Last Updated: November 29, 2025
Phospholipid Structures
Phospholipids are synthesized by esterification of an alcohol to the phosphate of phosphatidic acid (1,2-diacylglycerol 3-phosphate) or through the remodeling of lysophospholipids. Most phospholipids have a saturated fatty acid on C-1 (sn-1) and an unsaturated fatty acid on C-2 (sn-2) of the glycerol backbone. In humans, the fatty acids esterified to phosphatidic acid are most often 12 to 24 carbons in length and contain from 0 to 6 carbon-carbon double bonds. The most commonly added alcohols (serine, ethanolamine and choline) also contain nitrogen that may be positively charged, whereas, glycerol and inositol do not.
The major classifications of phospholipids are the phosphatidylserines (PS), the phosphatidylethanolamines (PE), the phosphatidylcholines (PC; often referred to as lecithins), the phosphatidylinositols (PI), phosphatidylglycerols (PG; major components of pulmonary surfactant), and the diphosphatidylglycerols (DPG; more commonly called the cardiolipins). As indicated in the paragraph above, the major phospholipids in these classes contain a saturated fatty acid at the C-1 (sn-1) position of the glycerol backbone and an unsaturated fatty acid at the C-2 (sn-2) position. The major human cardiolipin contains four molecules of linoleic acid as shown in the Figure below.
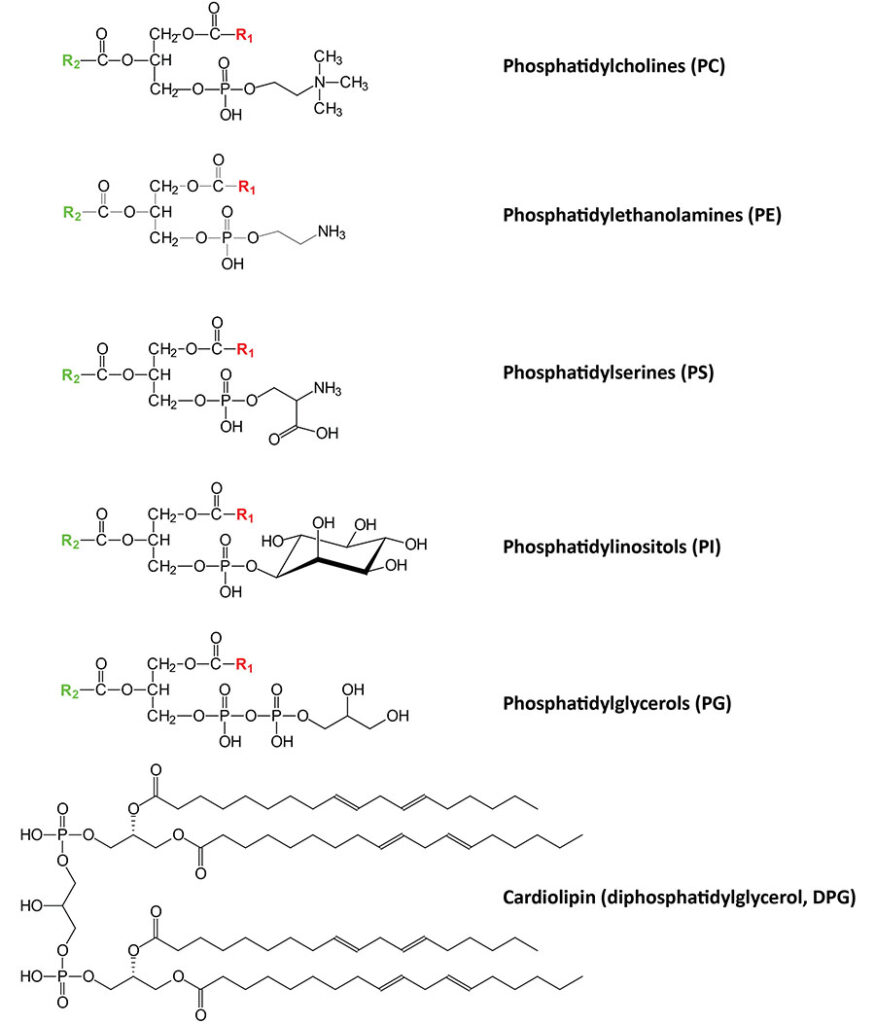
Table of Phospholipid Composition of Various Mammalian Membranes (% total phospholipid)
| Membrane Type | ||||||
| Phospholipid Class | Plasma | Mitochondrial | Nuclear | ER | Golgi | Lysosomal |
| Phosphatidylserine | 4% | 1% inner; 1% outer | 6% | 4% | 4% | 1% |
| Phosphatidylethanolamine | 21% | 38% inner; 34% outer | 25% | 21% | 17% | 21% |
| Phosphatidylcholine | 43% | 41% inner; 49% outer | 52% | 57% | 4% | 42% |
| Phosphatidylinositol | 4% | 2% inner; 9% outer | 4% | 9% | 9% | 6% |
| Cardiolipin | 0% | 16% inner; 5% outer | 0% | 0% | 0% | 0% |
Phospholipid Synthesis
Synthesis of the phospholipids occurs via a de novo pathway referred to as the Kennedy pathway which takes place within the endoplasmic reticulum, ER. This pathway involves the fatty acid esterification of glycerol-3-phosphate at the sn-1 and sn-2 positions generating a phosphatidic acid followed by addition of the polar head group.
The action of phospholipases generates lysophospholipids which can be re-esterified via the action of various enzymes of the lysophospholipid acyltransferase (LPLAT) family resulting in remodeling of phospholipids. This latter pathway to phospholipid synthesis is termed the Lands pathway.
Enzymes of the LPLAT family belong to two subfamilies of functionally related enzymes identified as the AGPAT family and the membrane bound O-acyltransferase domain containing (MBOAT) family. As discussed in the Synthesis of Triglycerides page, several AGPAT family enzymes are involved in the synthesis of triglycerides. The AGPAT family consists of 11 genes in humans and the MBOAT family consists of 8 genes in humans. Not all of these 19 genes encode enzymes involved in phospholipid synthesis. For example, the HHAT encoded hedgehog acyltransferase is an MBOAT family enzyme and it is responsible for the acylation of the secreted neurogenic protein sonic hedgehog.
Table of the Human Lysophospholipid Acyltransferase Family
| Official Gene Name | LysoPhosphoLipid AcylTransferase (LPLAT) Name | Other Names | Primary Tissues | Functions / Comments |
| AGPAT1 | LPLAT1 | LPAATα, LPAAT1 | ubiquitously expressed | localized to the endoplasmic reticulum (ER); involved in triglyceride synthesis in addition to phospholipid synthesis; plays a crucial role in the central nervous system and the reproductive system; polymorphisms in AGPAT1 gene associated with exfoliation syndrome |
| AGPAT2 | LPLAT2 | LPAATβ, LPAAT2 | highest in adipose tissue; 4-5-fold lower levels in kidney, liver, lung, intestines, skeletal muscle | localized to the endoplasmic reticulum (ER); involved in triglyceride synthesis in addition to phospholipid synthesis; play a crucial role in triglyceride production in adipose tissues; mutations in AGPAT2 gene associated with type 1 congenital generalized lipodystrophy (CGL) which is also known as Berardinelli–Seip lipodystrophy |
| AGPAT3 | LPLAT3 | LPAATγ, LPAAT3 | highest levels in kidney with brain being next highest; ubiquitously expressed | localized to the endoplasmic reticulum (ER), Golgi, and nuclear envelope; involved in triglyceride synthesis in addition to phospholipid synthesis; primarily utilizes docosahexaenoic acid (DHA, C22:6) for phospholipid synthesis |
| AGPAT4 | LPLAT4 | LPAATδ, LPAAT4 | highest levels in brain, 4-fold lower levels in several tissues | localized to the endoplasmic reticulum (ER); involved in triglyceride synthesis in addition to phospholipid synthesis; primarily utilizes unsaturated fatty acids as substrate including docosahexaenoic acid (DHA, C22:6), arachidonic acid (C20:4), linoleic acid (C18:2), and oleic acid (C18:1) |
| AGPAT5 | LPLAT5 | LPAATε, LPAAT5 | ubiquitously expressed with highest levels in brain and testis | localized to the endoplasmic reticulum (ER); involved in triglyceride synthesis in addition to phospholipid synthesis; preference for oleic acid (C18:1); polymorphisms associated with insulin resistance; experimental reductions in levels of enzyme shown to improve insulin sensitivity |
| LCLAT1 | LPLAT6 | AGPAT8, ALCAT1 | ubiquitously expressed with highest levels in the intestines | localized to the endoplasmic reticulum (ER); acylates numerous lysoPL substrates; exhibits preference for stearic acid (C18:0) and lysophosphatidylinositol (lysoPI); also utilizes lysophosphatidylglycerol (lysoPG) and lysocardiolipin (lysoCL) as substrates; is involved in oxidative stress and mitochondrial dysfunction via its role in cardiolipin remodeling |
| LPCAT1 | LPLAT8 | AGPAT9, AGPAT10 | lung, spleen | localized to the endoplasmic reticulum (ER) and lipid droplets (LD); primarily involved in the synthesis of phosphatidylcholines (PC); utilizes palmitic acid (C16:0) as a substrate; is involved in the synthesis of dipalmitoylphosphatidylcholine (DPPC; also termed dipalmitoyllecithin) which is a critical component of pulmonary surfactant; function of LPCAT1 may contribute to progression of numerous cancers |
| LPCAT2 | LPLAT9 | AGPAT11, LysoPAFAT | thyroid gland; 5-fold lower levels in many other tissues | localized to the endoplasmic reticulum (ER) and lipid droplets (LD); primarily responsible for the synthesis of platelet activating factor (PAF); utilizes acetic acid for incorporation into lyso-PAF; activity is dependent on Ca2+; only enzyme of this family that is regulated by phosphorylation |
| LPCAT3 | LPLAT12 | MBOAT5 | ubiquitously expressed with highest levels in the intestines with high levels also in liver | localized to the endoplasmic reticulum (ER); preference for linoleic acid (C18:2) and arachidonic acid (C20:4) when acylating lysophosphatidylcholine (lysoPC), lysophosphatidylethanolamine (lysoPE), and lysophosphatidylserine (lysoPS); plays an essential role in lipid mobilization in the gut and the liver; plays a role in the regulation of gut-brain communication through regulated gut hormone secretion; loss of LPCAT3 function is associated with lipid malabsorption |
| LPCAT4 | LPLAT10 | AGPAT7, LPEAT2 | ubiquitously expressed with highest levels in the stomach | localized to the endoplasmic reticulum (ER); exhibits broad substrate specificity and utilizes palmitic acid (C16:0), stearic acid (C18:0), and oleic acid (C18:1) in the production of phosphatidylethanolamines (PE), phosphatidylcholines (PC), and phosphatidylglycerols (PG); also acylates alkyl-lysoPC and alkenyl-lysoPE; may also utilize DHA as a substrate |
| LPGAT1 | LPLAT7 | FAM34A | ubiquitously expressed with highest levels in the small intestines and thyroid gland | localized to the endoplasmic reticulum (ER); primarily acylates lysophosphatidylglycerols (lysoPG) but is involved in the synthesis of numerous phospholipids; utilizes palmitic acid (C16:0), stearic acid (C18:0), and oleic acid (C18:1); polymorphisms in gene associated with susceptibility to obesity in Pima Indians |
| MBOAT1 | LPLAT14 | OACT1, LPEAT1 | ubiquitously expressed with highest levels in the stomach and intestines | localized to the endoplasmic reticulum (ER); preference for oleic acid (C18:1) but also utilizes palmitic acid (C16:0) and arachidonic acid (C20:4) |
| MBOAT2 | LPLAT13 | OACT2, LPEAT, LPAAT | ubiquitously expressed with highest levels in the brain and bone marrow | localized to the endoplasmic reticulum (ER); exhibits selectivity for incorporation of oleic acid (C18:1) into lysophosphatidylcholine (lysoPC) and lysophosphatidylethanolamine (lysoPE) |
| MBOAT7 | LPLAT11 | LENG4, LPIAT1 | ubiquitously expressed with highest levels in the adrenal gland, brain, and testis | localized to the endoplasmic reticulum (ER); LPIAT1 is lysophosphatidylinositol acyltransferase 1; exhibits selectivity for incorporation of arachidonic acid (C20:4) into lysophosphatidylinositol (lysoPI); loss of function is associated with abnormal brain development; polymorphisms in gene associated with hepatic steatosis |
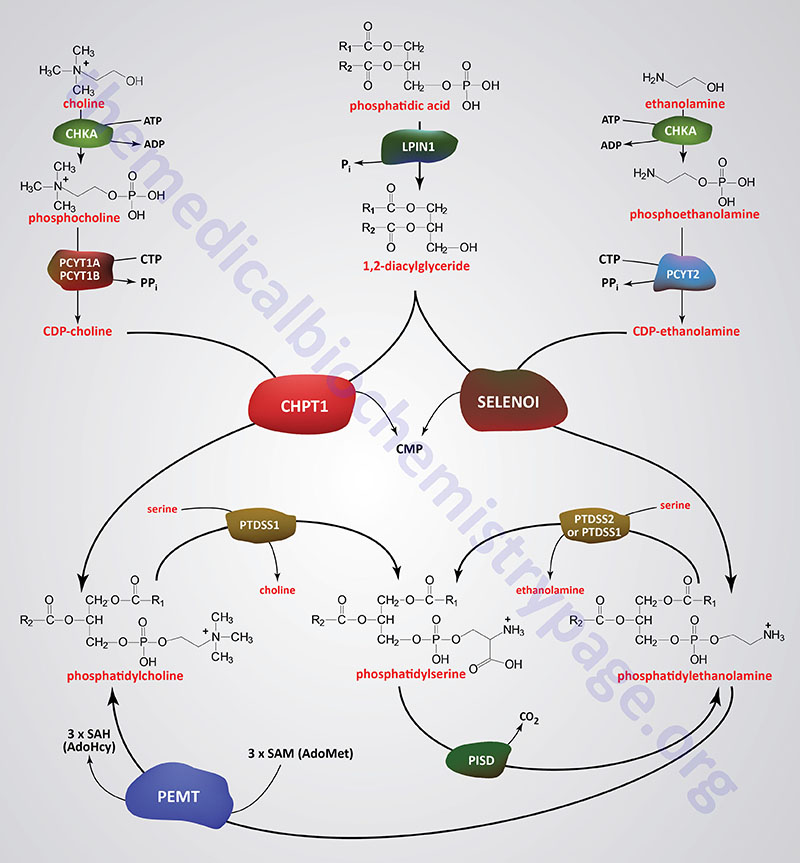
Synthesis of Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine
Phosphatidylcholines, PC
This class of phospholipid is also called the lecithins. The phosphatidylcholines represent the predominant phospholipid in the membranes of human cells, representing >50% of the phospholipid composition of membranes. At physiological pH, phosphatidylcholines are neutral zwitterions. They contain primarily palmitic (16:0) or stearic acid (18:0) at carbon 1 and primarily oleic acid (18:1), linoleic acid (18:2), or linolenic acid (18:3) at carbon 2.
The specific phosphatidylcholine with both C-1 and C-2 esterified with palmitic acid (16:0) is commonly identified as dipalmitoyllecithin. Dipalmitoyllecithin is a component of pulmonary surfactant and is the major (80%) phospholipid found in the extracellular lipid layer lining the pulmonary alveoli.
Phosphatidylcholine Synthesis
In humans, phosphatidylcholines (PC) are primarily synthesized via the CDP-choline pathway (Kennedy pathway). The choline required for the synthesis of phosphatidylcholines is primarily acquired in the diet. Indeed, one of the major fates of dietary choline is its incorporation into phosphatidylcholines. The role of choline in the synthesis of PC makes the compound essential in the diet. Indeed, when choline is restricted from the diet the result is development of fatty liver disease as well as muscle and liver damage. These pathologies can be reversed upon reintroduction of choline to the diet.
Dietary choline, as well as that recycled from phosphatidylcholines, is converted to betaine (trimethylglycine) which plays an important role in the synthesis of methionine from homocysteine and as a consequence, contributes to numerous methylation reactions when methionine is converted to S-adenosylmethionine (SAM or AdoMet). In addition to its role in the synthesis of betaine and PC, choline is an important constituent of the sphingomyelins. Choline is also acetylated forming the neurotransmitter, acetylcholine.
Dietary choline is transported into cells via the FLVCR1 (feline leukemia virus subgroup C receptor 1) and FLVCR2 encoded transporters. The transporters are members of the SLC family of transporters and as such are also identified as SLC49A1 and SLC49A2, respectively. Two isoforms of FLVCR1 have been characterized, FLVCR1a and FLVCR1b.
Choline is first activated by phosphorylation and then by coupling to CDP prior to attachment to a 1,2-diacylglycerol. The phosphorylation of choline is catalyzed by choline kinase-α (encoded by the CHKA gene). Humans express two choline kinase genes, CHKA and CHKB (choline kinase-β; formerly called choline kinase-like, CHKL). The CHKA encoded enzyme is also responsible for the phosphorylation and activation of ethanolamine.
Phosphocholine is then converted to CDP-choline by the enzymes of the cytidylyltransferase family. Humans express three genes in this family, PCYT1A (phosphate cytidylyltransferase 1, choline, alpha), PCYT1B (phosphate cytidylyltransferase 1, choline, beta), and PCYT2 (phosphate cytidylyltransferase 2, ethanolamine). The PCYT1A and PCYT1B encoded enzymes are involved in CDP-choline synthesis while the PCYT2 encoded enzyme is involved in CDP-ethanolamine synthesis. The PCYT1A enzyme contains a nuclear localization signal and thus, it is predominantly found in this organelle.
Phosphatidylcholines are then synthesized from CDP-choline and a 1,2-diacylglycerol with concomitant release of CMP. The 1,2-diacylglycerols are derived from the action of enzymes of the phospholipid phosphatase family, such as lipin-1 (encoded by the LPIN1 gene), phospholipid phosphatase 1 (encoded by the PLPP1 gene), phospholipid phosphatase 2 (encoded by the PLPP2 gene), and phospholipid phosphatase 3 (encoded by the PLPP3 gene). The last reaction of PC synthesis is catalyzed by diacylglycerol cholinephosphotransferase which is encoded by the CHPT1 (choline phosphotransferase 1) gene. The CHPT1 encoded enzyme is often referred to as CDP-choline:1,2-diacylglycerol cholinephosphotransferase.
Phosphatidylcholines can also be synthesized from CDP-choline and a DAG via the action of the CEPT1 (choline/ethanolamine phosphotransferase 1) encoded enzyme.
An additional pathway for the synthesis of PC involves the trimethylation of PE using S-adenosylmethionine (SAM; or AdoMet) as methyl group donor. This second PC synthesis pathway only occurs to a significant degree in hepatocytes and is catalyzed by phosphatidylethanolamine N-methyltransferase which is encoded by the PEMT gene. Indeed, this liver pathway represents a minor mechanism for the de novo synthesis of choline. The release of choline from PC is catalyzed by enzymes of the phospholipase D (PLD) family as well as in the conversion of PC to phosphatidylserine (PS) catalyzed by phosphatidylserine synthase 1 (encoded by the PTDSS1 gene).
Although an additional PC biosynthesis pathway occurs in some organisms involving the addition of choline to CDP-activated 1,2-diacylglycerol, this pathway is not known to occur in human cells.
The action of phospholipases of the PLA2 family on PC yields a family of bioactive lipids termed the lysophosphatidylcholines, LPC (also designated lysoPC). One of the PLA2 family member enzymes, commonly identified as iPLA2 (encoded by the PNPLA6 gene), deacetylates PC to yield glycerophosphocholine (GPC; also designated alpha-GPC). Glycerophosphocholine is also derived via the actions of lysosomal PLA2 (encoded by the PLA2G15 gene) acting on LPC in the lysosomes. This lysosomal GPC is transported to the cytosol by the transporter identified as battenin (encoded by the CLN3 gene). Mutations in the CLN3 gene are the cause of the neuronal ceroid lipofuscinosis commonly identified as Batten–Spielmeyer–Sjogren disease.
Glycerophosphocholine (GPC) represents a major intracellular storage form of choline, particularly in cholinergic neurons that synthesize acetylcholine (ACh) from choline and acetyl-CoA. Release of choline and glycerol-3-phosphate from GPC involves the actions of the enzyme, glycerophosphodiesterase 1, which is encoded by the GPCPD1 gene. Expression of the GPCPD1 gene is high in brain and skeletal muscle. Deficiency of skeletal muscle GPCPD1 is associated with a disruption in glucose homeostasis, similar to the effects of insulin resistance in skeletal muscle in type 2 diabetes. The glucose homeostatic disruption with deficiency in skeletal muscle GPCPD1 activity is not observed when the activity is inactivated in the liver or in adipose tissue.
Phosphatidylcholine Synthesis and Respiratory Distress Syndrome (RDS)
A significant cause of death in premature infants and, on occasion, in full term infants, is respiratory distress syndrome (RDS), also referred to as hyaline membrane disease. This condition is caused by an insufficient amount of pulmonary surfactant.
Under normal conditions pulmonary surfactant is synthesized by type II endothelial cells and is secreted into the alveolar spaces to prevent atelectasis (alveolar collapse) following expiration during breathing. Surfactant is comprised primarily of dipalmitoyllecithin. Additional lipid components include phosphatidylglycerol and phosphatidylinositol. In addition to the lipid composition, surfactant contains proteins of 18 kDa and 36 kDa (termed surfactant proteins).
During the third trimester the fetal lung synthesizes primarily sphingomyelin, and type II endothelial cells convert the majority of their stored glycogen to fatty acids and then to dipalmitoyllecithin. Fetal lung maturity can be determined by measuring the ratio of lecithin to sphingomyelin (L/S ratio) in the amniotic fluid. An L/S ratio less than 2.0 indicates a potential risk of RDS. The risk is nearly 75-80% when the L/S ratio is 1.5.
Phosphatidylethanolamines, PE
The phosphatidylethanolamines (PE) are neutral zwitterions at physiological pH. They contain primarily palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. Phosphatidylethanolamines are the second most abundant phospholipids in human cells representing between 15% and 25% of the total membrane phospholipid. Phosphatidylethanolamines are found primarily associated with the inner leaflet of the plasma membrane.
Phosphatidylethanolamine Synthesis
Synthesis of PE in humans is carried out by at least two distinct pathways and they account for the bulk of PE synthesis . These two pathways operate either in the ER (referred to as the Kennedy pathway) or in the mitochondria.
The Kennedy pathway of PE synthesis involves the phosphorylation of ethanolamine via the action of CHKA encoded enzyme described in the section above. Humans express two additional kinases that phosphorylate ethanolamine and these, unlike the CHKA encoded enzyme, are specific for ethanolamine. These enzymes are identified as ethanolamine kinase 1 (encoded by the ETNK1 gene) and ethanolamine kinase 2 (encoded by the ETNK2 gene). The ETNK1 encoded enzyme is localized to the cytosol and, therefore, does not contribute to the Kennedy pathway of PE synthesis.
Phosphoethanolamine is then converted to CDP-ethanolamine via the activity of the PCYT2 encoded enzyme whose activity is similar to the PCYT1A and PCYT1B encoded enzymes involved in the synthesis of phosphatidylcholines.
Formation of PE from CDP-ethanolamine and a 1,2-diacylglycerol is catalyzed by selenoprotein I which is encoded by the SELENOI gene. The encoded enzyme is more commonly referred to as CDP-ethanolamine:1,2-diacylglycerol ethanolamine phosphotransferase (EPT) and also as diacylglycerol ethanolamine phosphotransferase.
Phosphatidylethanolamines can also be synthesized from CDP-ethanolamine and a DAG via the action of the CEPT1 (choline/ethanolamine phosphotransferase 1) encoded enzyme.
The second major pathway for PE synthesis occurs in the inner mitochondrial membrane and involves the decarboxylation of PS. The decarboxylation reaction is catalyzed by phosphatidylserine decarboxylase which is encoded by the PISD gene. This mitochondrial reaction requires the transport of PS into the mitochondria from the ER via the transporter complex consisting of the PRELID3B and TRIAP1 encoded proteins.
The PRELI domain (PRELID) family of proteins are involved in the biosynthesis of phospholipids in the ER and in the mitochondria. PRELI refers to Proteins of Relevant Evolutionary and Lymphoid Interest domain containing family where PRELID3B is the PRELI Domain 3B gene. TRIAP1 refers to TP53 Regulated Inhibitor of APoptosis 1).
Another minor PE synthesis reaction that occurs in the ER membranes involves the fatty acylation of a lysophosphatidylethanolamine (referred to as the lyso-PE pathway) converting it to PE. This reaction is catalyzed by an enzyme called lysophospholipid acyltransferase 1 (LPEAT1). Lysophospholipid acyltransferase 1 is encoded by the MBOAT2 (membrane bound O-acyltransferase domain containing 2) gene. Lyso-PE molecules can also be converted to PE via the actions of the LPCAT3 (lysophosphatidylcholine acyltransferase 3) and LPCAT4 encoded enzymes. LPCAT3 was formerly identified as MBOAT5. LPCAT4 was formerly identified as LPEAT2 (lysophospholipid acyltransferase 2).
Phosphatidylserines, PS
Phosphatidylserines (PS) will carry a net charge of –1 at physiological pH and are composed of fatty acids similar to the phosphatidylethanolamines (PE).
Phosphatidylserine Synthesis
The pathway for PS synthesis involves base exchange reactions of serine for the ethanolamine in PE or serine for the choline in PC. These exchange reactions can be catalyzed by a single enzyme, phosphatidylserine synthase 1, which is encoded by the PTDSS1 gene. The enzyme encoded by the PTDSS1 gene exhibits a high degree of preference for PC and is, therefore, the primary enzyme for generation of PS via the base exchange reaction.
A related gene, PTDSS2, encodes phosphatidylserine synthase 2 and this enzymes appears to be exclusive for the exchange of serine for ethanolamine in PE generating PS.
Also, as indicated below, PS can serve as a source of PE synthesis in the mitochondria through a decarboxylation reaction catalyzed by the PISD encoded enzyme.
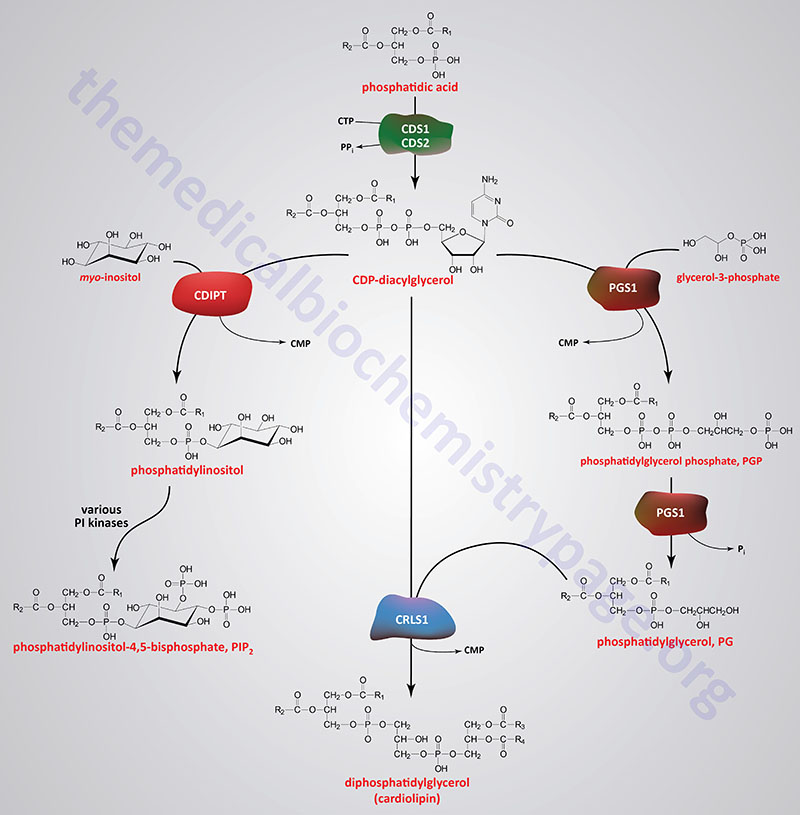
Synthesis of Phosphatidylinositols, Phosphatidylglycerols, and Cardiolipins
Phosphatidylinositols (PI or PtdIns)
The phosphatidylinositols contain almost exclusively stearic acid at carbon 1 and arachidonic acid at carbon 2. Phosphatidylinositols composed exclusively of non-phosphorylated inositol exhibit a net charge of –1 at physiological pH. Phosphatidylinositols exist in membranes with various levels of phosphate esterified to the hydroxyls of the inositol. Molecules with phosphorylated inositol are termed phosphoinositides and those that contain inositol with more than one phosphate are referred to as polyphosphoinositides.
The phosphoinositides are important intracellular transducers of signals emanating from the plasma membrane. The predominant signal transducing polyphosphoinositides are the phosphatidylinositol-4,5-bisphosphates (PIP2; also designated PtdIns-4,5-P2).
Phosphatidylinositol Synthesis
In humans, the synthesis of a phosphatidylinositol, PI (as well as a PG), begins with the formation of CDP-diacylglycerol (CDP-DAG) from phosphatidic acid (PA) and CTP. The synthesis of CDP-DAG is catalyzed by at least four enzymes that includes CDP-diacylglycerol synthase 1 and CDP-diacylglycerol synthase 2, which are encoded by the CDS1 and CDS2 genes, respectively. In humans the major enzyme responsible for the synthesis of CDP-DAG used for PI synthesis is likely to be the CDS2 encoded enzyme.
A third enzyme that can generate CDP-DAG is a bifunctional enzyme that is primarily responsible for the condensation of a CDP-DAG with myo-inositol forming a PI and CMP. This latter enzyme is called CDP-diacylglycerol–inositol 3-phosphatidyltransferase and it is encoded by the CDIPT gene. The CDIPT encoded enzyme is also referred to simply as phosphatidylinositol synthase, PIS. In the reverse direction, the generation of CDP-DAG by CDIPT, which is dependent on CMP, is believed to function to reduce the cellular content of PI and, as such, is not considered a significant pathway for the generation of CDP-DAG to be utilized in phospholipid synthesis.
Another CDP-DAG synthesizing enzyme is localized to the inner mitochondrial membrane. This mitochondrial enzyme is responsible for the synthesis of the CDP-DAG that is utilized for the synthesis of the mitochondrial phosphatidylglycerols (PG) and ultimately the cardiolipins. This mitochondrial enzyme is encoded by the TAMM41 gene.
Phosphatidylinositols subsequently undergo a series of phosphorylations of the hydroxyls of inositol leading to the production of polyphosphoinositides. One particular polyphosphoinositide, phosphatidylinositol-4,5-bisphosphate (PIP2; also designated PtdIns-4,5-P2), is a critically important membrane phospholipid involved in the transmission of signals for cell growth and differentiation.
Another critically important PI is phosphatidylinositol-3,4,5-trisphosphate (PIP3; also designated PtdIns-3,4,5-P3) which is generated from PIP2 via the action of enzymes of the phosphatidylinositol-3-kinase, PI3K family. PIP3 activates the kinase called 3-phosphoinositide-dependent kinase-1 (PDPK1) which serves as a master regulatory kinase in the activation of the kinases PKB/AKT, PKC, S6K (p70-S6 kinase 1), and the Ser/Thr kinases identified as SGK1, SGK2, and SGK3. PKB/AKT was originally identified as the tumor inducing gene in the AKT8 retrovirus found in the AKR strain of mice. Humans express three genes in the AKT family identified as AKT1 (PKBα), AKT2 (PKBβ), and AKT3 (PKBγ).
Phosphatidylglycerols, PG
Phosphatidylglycerols exhibit a net charge of –1 at physiological pH. These molecules are found in high concentration in mitochondrial membranes and as components of pulmonary surfactant.
Phosphatidylglycerol Synthesis
Phosphatidylglycerols are primarily synthesized within the mitochondria following the transport of phosphatidic acid (PA) into the mitochondria from the ER. Phosphatidic acids are transported into the mitochondria via the action of the transporter complex composed of PRELID1 (PRELI domain containing 1) and TRIAP1.
Phosphatidylglycerols are also synthesized in membranes of the ER in a two-step process that begins with CDP-diacylglycerol (CDP-DAG) and glycerol-3-phosphate. The first reaction yields phosphatidylglycerol phosphate and this reaction is catalyzed by the enzyme identified as phosphatidylglycerophosphate synthase 1 (encoded by the PGS1 gene). The CDP-DAG is synthesized by the CDS1, CDS2, or CDIPT encoded enzymes as described in the previous section. The PGS1 encoded enzyme is localized to both the ER membrane and the inner mitochondrial membrane.
Phosphatidylglycerol phosphates are then converted to phosphatidylglycerols (PG) via removal of phosphate most likely by the action of the PGS1 encoded enzyme although the activity has been called PGP phosphatase, phosphatidylglycerol phosphate phosphatase, and phosphatidylglycerophosphatase. Phosphatidylglycerols are also the precursors for the synthesis of the diphosphatidylglycerols (DPG) which are more commonly called the cardiolipins. The cardiolipins are major lipid components of the inner mitochondrial membrane.
Cardiolipins (Diphosphatidylglycerols):
The cardiolipins are very acidic, exhibiting a net charge of –2 at physiological pH. They are found almost exclusively within the inner mitochondrial membrane. Cardiolipins are enriched in cardiac and skeletal muscle mitochondria. The cardiolipins are synthesized within the mitochondria, as discussed in the next section, by the condensation of a CDP-diacylglyceride (CDP-DAG) with a phosphatidylglycerol (PG) in a reaction catalyzed by cardiolipin synthase 1 which is encoded by the CRLS1 gene.
Mitochondrial Phospholipid Synthesis: Role of Acylglycerol Kinase
Acylglycerol kinase is a phospholipid kinase that is primarily localized to the mitochondrial inner membrane where it functions in mitochondrial phospholipid homeostasis. Acylglycerol kinase is also identified as multi-substrate lipid kinase (MULK). The identification of AGK came about through an investigation of the underlying cause of Sengers syndrome. Sengers syndrome is a disorder that is characterized by hypertrophic cardiomyopathy, cataracts, and lactic acidosis, with or without skeletal myopathy.
Acylglycerol kinase catalyzes the synthesis of mitochondrial lysophosphatidic acid (LPA) and phosphatidic acid (PA) from monoacylglycerides (MAG) and diacylglycerides (DAG), respectively. The PA product of AGK is the substrate for the TAMM41 (TAM41 mitochondrial translocator assembly and maintenance homolog) encoded protein which utilizes CTP to generate CDP-diacylglycerides (CDP-DAG).
As indicated above, phosphatidic acids (PA) can also be transported into the mitochondria via the action of the transporter complex that consists of the PRELID1 and TRIAP1 encoded proteins.
The CDP-DAG can then be converted to phosphatidylglycerophosphate (PGP) via the action of the PGS1 encoded enzyme. PGP is dephosphorylated by the PTPMT1 (protein tyrosine phosphatase mitochondrial 1) encoded enzyme generating phosphatidylglycerides, PG. Cardiolipin synthase 1 will then convert CDP-DAG and PG to immature cardiolipins (CL) with the release of CMP.
The products of the cardiolipin synthase 1 reaction require further modification to become fully functional. The immature cardiolipins are substrates for the phospholipid-lysophospholipid transacylase encoded by the TAFAZZIN gene. The TAFAZZIN encoded enzyme carries out remodeling of the fatty acids attached to the four glycerol hydroxyls of a cardiolipin such that the predominant mature cardiolipins contain four molecules of linoleic acid. The use of name TAFAZZIN comes from the name of a masochistic character (Tafazzi) in a popular Italian comedy reflecting the difficulty researchers had with identifying the function of the gene.
The TAFAZZIN gene is located on the X chromosome (Xq28). Expression of the TAFAZZIN gene is highest in cardiac and skeletal muscle cells. Mutations in the TAFAZZIN gene are associated with Barth syndrome which is characterized by cardiomyopathy, skeletal muscle weakness, neutropenia, and abnormal growth.
The significance of the activity of AGK to overall mitochondrial function is that phospholipids, particularly cardiolipins, in the membranes of these organelles are involved in the regulation of mitochondrial apoptosis and autophagy.
Acylglycerol kinase is encoded by the AGK gene. The AGK gene is located on chromosome 7q34 and is composed of 18 exons that generate two alternatively spliced mRNAs encoding precursor proteins of 422 amino acids (isoform 1) and 350 amino acids (isoform 2). Expression of the AGK gene is ubiquitous with the highest levels of expression found in the intestines, kidney, and brain.
Mitochondrial membranes also contain phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidylinositols (PI). The synthesis of PE from PS is catalyzed by the PISD encoded phosphatidylserine decarboxylase.
Cytosolic PS is transported into the mitochondria via the action of PRELID3B/TRIAP1 transporter complex.
Cytosolic PC is transported into the mitochondria via the action of the STARD7 (StAR related lipid transfer domain containing 7) encoded transporter.
In addition to its kinase activity, AGK serves as a component of the translocase of the mitochondrial inner membrane 22 (TIM22) complex and in this complex it facilitates protein anchoring to the mitochondrial inner membrane. This function of AGK does not involve its kinase function. Additional non-kinase activities have been found to be associated with AGK that involve its localization to the plasma membrane and to the cytosol. When localized in the plasma membrane AGK associates with the phosphoinositide phosphatase, PTEN. When AGK is associated with PTEN the phosphatase activity of PTEN is inhibited which allows the PI3K, AKT/PKB, and mTORC1 signal transduction pathways to remain active.
Mitochondrial membranes also contain ceramides, the synthesis of which is detailed in the Sphingolipid Metabolism and the Ceramides page.
Phospholipases and Phospholipid Remodeling
The fatty acid distribution at the C–1 and C–2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. Phospholipid turnover results from the action of numerous different phospholipases and phospholipid phosphatases. There are various phospholipases that exhibit substrate specificities for different positions in phospholipids.
In many cases the acyl group which was initially transferred to glycerol, by the action of the acyl transferases, is not the same acyl group present in the phospholipid when it resides within a membrane. The remodeling of acyl groups in phospholipids is the result of the action of enzymes of the phospholipase A1 (PLA1) and phospholipase A2 (PLA2) families. The products of the PLA1 and PLA2 catalyzed reactions are termed lysophospholipids (LPL or lysoPL).
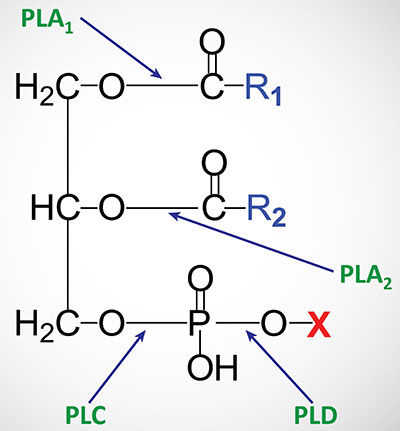
Various lysophospholipids (lysoPL) exhibit biological activities as detailed in the Bioactive Lipids and Lipid Sensing Receptors page as well as the Signal Transduction Pathways: Phospholipids page. In addition to exhibiting biological activities, lysoPL represent an intermediate in the remodeling process of phospholipids. The various lysoPL can be re-esterified by enzymes of the lysophospholipid acyltransferase (LPLAT) family to generate phospholipids with different fatty acid compositions. This pathway of phospholipid synthesis is referred to as the Lands pathway. As discussed in the Synthesis of Triglycerides page, and outlined in the Table above, humans express 14 genes encoding enzymes of the LPLAT family which includes enzymes also classified as AGPATs and membrane bound O-acyltransferases (MBOATs).
Outside of the context of phospholipid remodeling, PLA2 is an important enzyme whose activity is responsible for the release of polyunsaturated fatty acids (PUFA), such as arachidonic acid, from the C-2 (sn-2) position of membrane phospholipids. The released arachidonate is then a substrate for the synthesis of the eicosanoids and many other bioactive lipids of the oxylipin family. In fact, there is not just a single PLA2 enzyme. At least 30 enzymes have been identified with PLA2 activity. For more details on the PLA2 family of phospholipases visit the Signal Transduction Pathways: Phospholipids page.
There are 10 isozymes that are in the secretory pathway and these PLA2 isozymes are abbreviated sPLA2. These secretory enzymes are low molecular weight proteins that are Ca2+-dependent and are involved in numerous processes including modification of eicosanoid generation, host defense, and inflammation.
The cytosolic PLA2 family (cPLA2) comprises three isozymes with cPLA2α being an essential component of the initiation of arachidonic acid metabolism. Like the sPLA2 enzymes, the cPLA2 enzymes are tightly regulated by Ca2+. In addition, this class of PLA2 enzyme is regulated by phosphorylation. An additional family of two PLA2 isozymes that are Ca2+-independent for activity are identified as iPLA2. This latter class of enzyme is involved primarily with the remodeling of phospholipids.
Finally, a class of PLA2 enzymes, whose original member was identified as being involved in the hydrolysis and inactivation of platelet activating factor, PAF (see the section below), contains at least four members. The original activity was called PAF-acetylhydrolase (PAF-AH). The PAF hydrolyzing PLA2 isozymes are Ca2+-independent like the iPLA2 family. Because this latter family was shown to not only hydrolyze PAF but also oxidized phospholipids and to be associated with lipoprotein particles in the circulation they are identified as the lipoprotein-associated PLA2 (Lp-PLA2) family. More details on the functions of the Lp-PLA2 family of enzymes can be found in the Lipoproteins, Blood Lipids, and Lipoprotein Metabolism page.
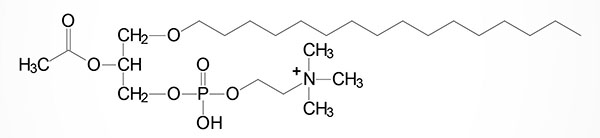
Plasmalogens
Plasmalogens are glycerol ether phospholipids. They are of two types, alkyl ether (–O–CH2–) and alkenyl ether (–O–CH=CH–). Dihydroxyacetone phosphate serves as the glycerol precursor for the synthesis of glycerol ether phospholipids. Three major classes of plasmalogens have been identified: choline, ethanolamine and serine plasmalogens. Ethanolamine plasmalogen is prevalent in myelin. Choline plasmalogen is abundant in cardiac tissue.
The initiation of plasmalogen synthesis takes place inside the lumen of the peroxisome using dihydroxyacetone phosphate (DHAP) as the building block. The first enzyme in the synthesis of plasmalogens is glycerone phosphate O-acyltransferase encoded by the GNPAT gene (also known as dihydroxyacetone phosphate acyltransferase, DHAPAT). The GNPAT encoded enzyme adds a fatty acyl group to the sn-1 position of DHAP.
The GNPAT gene is located on chromosome 1q42.2 and is composed of 16 exons that generate two alternatively spliced mRNAs encoding proteins of 680 amino acids (isoform 1) and 619 amino acids (isoform 2).
The next step in the synthesis pathway is catalyzed by alkylglycerone phosphate synthase encoded by the AGPS gene. The AGPS encoded enzyme exchanges the acyl group added by GNPAT for an alkyl group. The alky-DHAP is then reduced by a reductase found in both the peroxisomes and the ER.
The AGPS gene is located on chromosome 2q31.2 and is composed of 22 exons that encode a 658 amino acid precursor protein.
The remaining reactions of plasmalogen synthesis occur within the ER and include acylation reactions at the sn-2 position and the removal of the phosphate group by one of the phosphatidic acid phosphatase family of enzymes.
One particular type of choline plasmalogen (1-O-1′-enyl-2-acetyl-sn-glycero-3-phosphocholine) has been identified as an extremely powerful biological mediator, capable of inducing cellular responses at concentrations as low as 10–11M. This molecule is called platelet activating factor, PAF. PAF functions as a mediator of hypersensitivity, acute inflammatory reactions and anaphylactic shock. PAF is synthesized in response to the formation of antigen-IgE complexes on the surfaces of basophils, neutrophils, eosinophils, macrophages and monocytes. The synthesis and release of PAF from cells leads to platelet aggregation and the release of serotonin from platelets. PAF also produces responses in liver, heart, smooth muscle, and uterine and lung tissues.
Clinical significance of the plasmalogen biosynthesis pathway is evidenced by the severe phenotypes associated with deficiencies in enzymes of the pathway. Deficiencies in both GNPAT and AGPS result in the peroxisomal disorders of the rhizomelic chondrodysplasia punctata (RCDP) family. These disorders are identified as RCDP1, RCDP2, RCDP3, RCDP4, and RCDP5.
The various RCDP disorders are characterized by disproportionately short stature primarily affecting the proximal parts of the extremities, a typical facial appearance including a broad nasal bridge, epicanthus, high-arched palate, dysplastic external ears, and micrognathia, congenital contractures, characteristic ocular involvement, dwarfism, and severe intellectual impairment with spasticity.
Almost all RCDP infants die within the first year of life. The most commonly occurring RCDP is RCDP1. Whereas RCDP1 and RCDP5 are classified as peroxisomal biogenesis disorders, RCDP2, RCDP3, and RCDP4 are classified as single peroxisomal enzyme deficiencies. Mutations in the PEX7 gene are the causes of RCDP1 and mutation in the PEX5 gene are the causes of RCDP5. Mutations in the GNPAT
