Last Updated: December 19, 2022
Introduction to CPT2 Deficiency
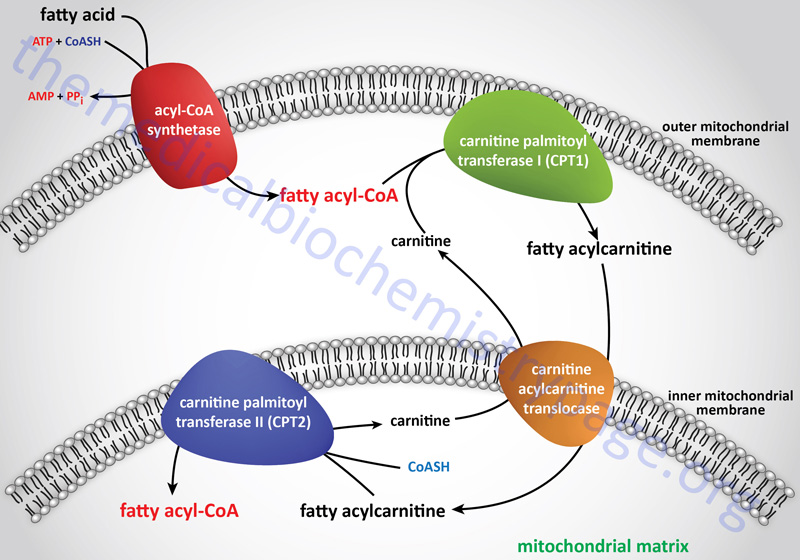
Carnitine palmitoyltransferase 2 (CPT2 or CPT-II) is one of a family of carnitine acyltransferases in humans that catalyze the reversible transfer of acyl groups between coenzyme A (CoASH) and L-carnitine, converting fatty acyl-CoA esters into fatty acyl-carnitine esters. The role of CPT2 is in the mitochondrial β-oxidation of long-chain fatty acids. Specifically CPT2, which is localized to the inner mitochondrial membrane, exchanges CoASH onto long-chain acyl-carnitines releasing free carnitine and generating a fatty acyl-CoA.
These CPT2 generated fatty acyl-CoA molecules can then serve as substrates for the inner mitochondrial membrane localized enzyme, very long-chain acyl-CoA dehydrogenase (VLCAD). Although humans contain a gene (ACADL) that encodes a long-chain acyl-CoA dehydrogenase (LCAD) enzyme, expression of this gene in humans is poor and the encoded enzyme does not contribute to mitochondrial long-chain fatty acid β-oxidation. Indeed, patients who were originally described as suffering from deficiencies in the LCAD enzyme were, in fact, found to be harboring mutations in the ACADVL gene which encodes the VLCAD enzyme.
Molecular Biology of CPT2 Deficiency
The CPT2 gene is located on chromosome 1p32.3 and consists of 5 exons that generate two alternatively spliced mRNAs. These mRNAs encode precursor proteins of 658 amino acids (isoform 1) and 635 amino acids (isoform 2).
Mutations in the CPT2 gene result in a group of disorders referred to as CPT2 deficiency syndromes. These disorders are inherited in an autosomal recessive manner. However, there are cases of individuals exhibiting symptoms even though they are heterozygous for a single mutant allele. Mutations that cause CPT2 deficiency syndromes include frame-shift and point mutations. No deletions or duplications have been found to be associated with inheritance of CPT2 deficiency.
In addition to CPT2, humans express three additional carnitine acyltransferases: carnitine palmitoyltransferase 1 (CPT1 or CPT-I), carnitine O-acetyltransferase (CRAT), and carnitine octanoyltransferase (CROT). Humans actually express three CPT1 genes identified as CPT1A, CPT1B, and CPT1C. The CPT1A encoded protein is localized to the outer mitochondrial membrane where it exchanges carnitine onto long-chain fatty acyl-CoAs releasing the CoASH and generating a long-chain fatty acylcarnitine.
Clinical Features of CPT-2 Deficiency
Inherited deficiencies of CPT2 result in disorders that are divided into three clinical phenotypes according to the age of presentation. These three forms are a lethal neonatal form, severe infantile form involving the liver, heart, and skeletal muscle (referred to as the infantile hepatocardiomuscular form), and a myopathic form that exhibits a range of age of onset from infancy to adulthood.
Lethal Neonatal CPT2 Deficiency
The lethal neonatal form of CPT2 deficiency is characterized by hypoketotic hypoglycemia, cardiomyopathy, liver failure, and respiratory distress. Infants born with this form of the disorder present with hepatic calcifications and cystic dysplastic kidneys. Neuronal migration defects are also found resulting in cystic dysplasia of the basal ganglia. Additional symptoms seen in neonates with this form of CPT2 deficiency include hydrocephalus, cerebral calcifications, agenesis of the corpus callosum, cerebellar vermian hypoplasia, polymicrogyria, pachygyria, as well as other neuronal migration defects. Characteristic blood work in this form of CPT2 deficiency shows reduced serum concentrations of total and free carnitine, and increased serum concentrations of long-chain acylcarnitines and lipids. Infants with this form of CPT2 deficiency usual die within days to months after birth. The most common mutations in the CPT2 gene associated with the lethal neonatal form are null mutations that lead to truncated proteins or to mRNA degradation.
Hepatocardiomuscular CPT2 Deficiency
Infants with the hepatocardiomuscular form of CPT2 deficiency present with episodic hypoketotic hypoglycemia. The overall clinical presentation of the hepatocardiomuscular form of CPT2 deficiency in infants is severe and includes cardiomyopathy and quite often is associated with sudden death. One particular mutation in the CPT2 gene, the mutation of a phenylalanine at amino acid position 352 for cysteine (identified as the F352C mutation) has been associated with an increased likelihood for sudden death in infancy. Individuals with the infantile hepatocardiomuscular form of the disease are often compound heterozygotes.
Adult Myopathic CPT-2 Deficiency
The adult form of CPT2 deficiency, the myopathic form, is the most common form of CPT2 deficiency. The myopathic form of CPT2 deficiency is also the most common disorder of lipid metabolism that affects skeletal muscle as well as the most common cause of inherited myoglobinuria. This form of CPT2 deficiency is characterized by recurrent episodes of muscle pain, rhabdomyolysis and myoglobinuria that is triggered by prolonged exercise.
Metabolic studies done in patients with adult myopathic CPT2 deficiency will show that oxidation of palmitic acid is normal at rest but severely impaired during prolonged low-intensity exercise. Palmitic acid is a 16 carbon saturated fatty acid representing the most abundant fatty acid in human blood. Often the symptoms of the myopathic form of CPT2 deficiency are not apparent until an adolescent undertakes endurance training such as for track in middle school. The myopathic form of CPT2 deficiency is a common cause of rhabdomyolysis and myoglobinuria in adults.
Biopsy tissue taken from skeletal muscle will show lipid accumulation when analyzed with stains such as Sudan black or oil red O. Analysis of CPT2 function in these biopsy samples will show reduced levels of activity. The capacity to oxidize medium-chain fatty acids via the mitochondrial β-oxidation pathway is partially preserved in patients with CPT2 deficiency due to the lack of a strict requirement for medium-chain fatty acids to be attached to carnitine for entry into the mitochondrial matrix. In patients with the myopathic form of CPT2 deficiency the utilization of medium-chain triglycerides results in dicarboxylic aciduria indicative of increased microsomal metabolism of fatty acids due to the limited capacity for mitochondrial fatty acid uptake in the absence of adequate CPT2 function. The most common (60%) mutation in the CPT2 gene observed in patients with the myopathic form of the disease is where the Ser at amino acid position 113 is mutated to a Leu (identified as the S113L mutation)
Treatment of CPT2 Deficiency
Clinical intervention in patients with CPT2 deficiency includes reducing the intake of long-chain fatty acids while still ensuring adequate intake of the essential fatty acids, linolenic and linoleic acid. Approximately one third of the caloric intake by these patients should be from medium-chain triglycerides (MCT). Patients with CPT2 deficiency should also follow a carbohydrate-rich diet while avoiding prolonged fasting. Carnitine supplementation will reduce the accumulation of toxic long-chain fatty acyl-CoAs.
Some therapeutic benefit has been demonstrated in patients with mild forms CPT-2 deficiency with the use of the fibrate drug, bezafibrate. In these patients the use of bezafibrate is associated with increased CPT2 gene expression and normalization of CPT-2 activity. The incidence of rhamdomyolysis was reduced in patients taking bezafibrate and their ability to undertake physical activity was greatly improved.
Another avenue of therapy for myopathic CPT2 deficiency patients is the inclusion of triglycerides with odd-chain fatty acids in their diets. The artificially produced triglyceride is called triheptanoin and it contains three seven-carbon saturated fatty acids. The released odd chain fatty acids provide anaplerotic substrates for the TCA cycle so this treatment is referred to as the anaplerotic diet. In addition to being used in the treatment of CPT2 deficiency, triheptanoin has been used in patients with pyruvate carboxylase deficiency and with VLCAD deficiency. Metabolism of the seven-carbon fatty acids in triheptanoin also results in increased production of ketones (β-ketopentanoate and β-hydroxypentanoate) which easily cross the blood-brain barrier (BBB) and are utilized by neural tissues.