Last Updated April 11, 2024
Introduction to Lafora Disease
Lafora disease is a fatal autosomal recessive disease that is the result of a defect in glycogen metabolism. The disease gets its name from the Spanish neuropathologist, Gonzalo Rodriguez Lafora, who initially characterized the disorder. Lafora disease is characterized by the presence of large insoluble glycogen aggregates, called Lafora bodies, in the cytoplasm of the cells of numerous tissues including neurons, heart, skeletal muscle, and liver. Lafora bodies are also known as polyglucosan bodies (PGB).
Essentially Lafora bodies are found in all tissues that form glycogen while the pathology of Lafora disease results from the massive accumulation of precipitated glycogen in neuronal cell bodies. Lafora disease is a late-onset neurodegenerative disease associated with myoclonus epilepsy (irregular and uncontrollable jerks of a muscle or group of muscles associated with epileptic seizures) and death within a few years of diagnosis.
The accumulation of Lafora bodies (PGB) results in aberrant glycosylation in the brain. Brain PBG are primarily composed of glucose since they are glycogen aggregates, but they react with other carbohydrates such as N-acetylglucosamine (GlcNAc), galactose, and fucose.
Molecular Biology of Lafora Disease
Loss-of-function mutations in either of two genes are the cause of Lafora disease. One gene (EPM2A) encodes the EPM2A glucan (glycogen) phosphatase, which is commonly called laforin (also known as laforin glycogen phosphatase). The EPM2A of the gene name refers to Epilepsy, Progressive Myoclonus type 2A. The other gene (NHLRC1) encodes the NHL repeat containing E3 ubiquitin protein ligase 1, which is commonly called malin. The NHLRC1 gene was also known as EPM2B. The term NHL repeat refers to the initial letter of the three Caenorhabditis elegans genes in which the repeat was originally characterized: Ncl-1 (abnormal NuCLeoli-1), 5-HT2A (serotonin receptor 2A), and Lin-41 (LINeage variant 41). Mutations in EPM2A account for 50% of Lafora disease cases and mutations in NHLRC1 account for the other 50% of cases.
The EPM2A gene is located on chromosome 6q24.3 and is composed of 15 exons that generate nine alternatively spliced mRNAs that collectively encode six distinct protein isoforms. Laforin isoform a is the longest of the EPM2A encoded proteins and is a 331 amino acid protein localized to the rough ER. Laforin contains an N-terminal carbohydrate-binding domain and a C-terminal dual-specificity phosphatase (DSP) domain.
The NHLRC1 gene is an intronless gene located on chromosome 6p22.3 that encodes a 395 amino acid single subunit ubiquitin E3 ligase. The malin protein contains a RING (Really Interesting New Gene) finger domain that harbors the E3 ubiquitin ligase activity and six NHL repeats.
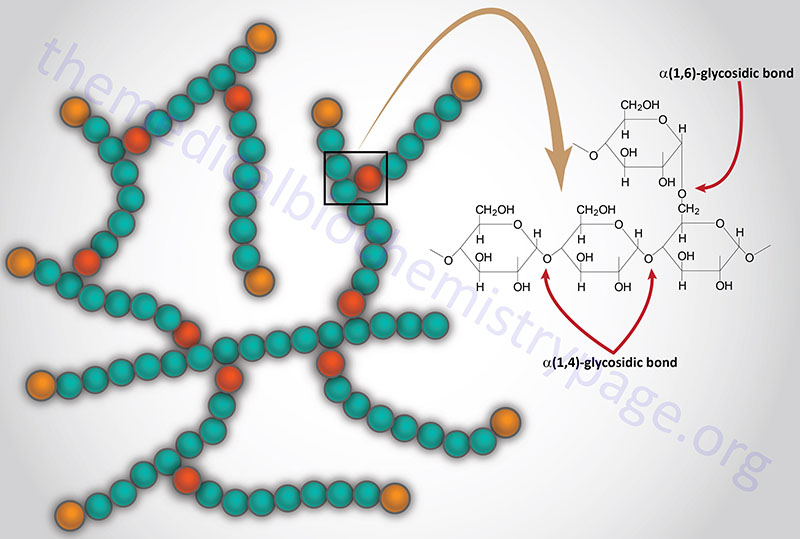
Laforin is a dual specificity phosphatase that removes phosphate from carbohydrates, primarily glycogen. The lack of laforin activity in Lafora disease leads to hyperphosphorylation of glycogen. Hyperphosphorylated glycogen disrupts the processes of branching and debranching leading to longer glucose chains than in a normal glycogen molecule. These abnormal glycogens (called polyglucans) are insoluble and ultimately lead to the formation of Lafora bodies.
Although normal glycogen has small amounts of phosphate, as phosphomonoesters, at positions C2, C3, and C6 of the glucose residues, Lafora bodies contain an increased proportion of these phosphomonoester groups on the glucose subunits of the polyglucans. The incorporation of phosphate into glycogen is a function of glycogen synthase which is capable of transferring the β-phosphate of UDP-glucose to glycogen. Normal laforin functions to maintain the low level of phosphomonoesters in glycogen which prevents the molecule from precipitating.
As its gene name implies, malin is a ubiquitin ligase whose precise function in the process of glycogen dephosphorylation is incompletely understood. However, evidence indicates that malin ubiquitylates the regulatory subunit of protein phosphatase 1 (PP1) which was originally identified as PTG for protein targeting glycogen. Several PTG encoding genes have been identified, all of which encode regulatory subunits of PP1. Malin has been shown to ubiquitylate PPP1R3A (the version of PTG found in skeletal muscle) and PPP1R3D. The malin mediated ubiquitylation occurs in a laforin-dependent manner. Together laforin and malin interact to regulate glycogen phosphorylation and chain length pattern, the latter of which is crucial to the solubility of glycogen in the cell.
Experiments have found that malin interacts with at least 22 proteins many of which are involved in overall glycogen metabolism. In addition to laforin and the PPP1R3A encoded protein, malin interacts with glycogen synthase encoded by the GSY1 gene (expression enriched in skeletal muscle although widely expressed), the muscle predominant form of glycogenin (encoded by the GYG1 gene), muscle glycogen phosphorylase (encoded by the PYGM gene), glycogen debranching enzyme (encoded by the AGL gene), and starch-binding domain-containing protein 1 (encoded by the STBD1 gene). The STBD1 encoded protein is a member of the family 20 carbohydrate binding module (CBM20) group of proteins that are involved in glycogen metabolism and autophagy.
The interaction of glycogen with glucosamine is facilitated by glycogen synthase, whereas the release of glucosamine from glycogen is facilitated by glycogen phosphorylase. Within the brain, glycogen serves a critical function as a storage molecule for not only glucose but also glucosamine required for protein N-glycosylation. In glycogen storage diseases, and specifically in Lafora disease, PGB sequester glucosamine, resulting in a decrease in the available pool of glucosamine that is required for the synthesis of UDP-GlcNAc. This PGB-mediated sequestration of glucosamine leads to significant pathology such as that seen in Lafora disease.
As indicated, the mutations that result in Lafora disease occur equally within the EPM2A and NHLRC1 genes. There does not appear to be a significant clustering of mutations to a particular region or domain of either gene that are associated with Lafora disease. Mutations that are found within the DSP of the laforin protein appear to compromise other properties of laforin that include subcellular localization, glycogen binding, and interaction with malin.
Clinical Features of Lafora Disease
The typical Lafora disease patient will begin to manifest symptoms in the second decade of life. The onset of symptoms is accompanied with vague symptoms of headaches and difficulties concentrating. Shortly after the onset of symptoms, myoclonus appears along with generalized tonic-clonic seizures. Tonic-clonic seizures were originally referred to as grand mal seizures, a term no longer used. In these types of seizures the term tonic refers to rigidity of the entire body and clonic refers to uncontrolled jerking. Patients commonly develop visual hallucinations.
In the first few years after symptoms begin to appear, Lafora disease patients will experience behavioural changes,
confusion, depression, dysarthria (difficulty speaking due to loss of control of the muscles used for speech), ataxia, and a decline of intellectual capacity.
The seizures, and especially the myoclonus, become intractable in Lafora disease patients. Atypical and myoclonic absences set in and they become so constant that a patient’s every thought and spoken words are constantly interrupted and incomplete. These attacks are both photosensitive and highly affected by emotion. Within a short period of a few years the patients are unable to attend school or work and become unable to walk because of frequent myoclonic and atonic attacks.
Most Lafora patients will die in status epilepticus (defined as a continuous seizure lasting more than 30 minutes, or two or more seizures without full recovery of consciousness between any of them) or from aspiration pneumonia secondary to the loss of neurological control of secretions.
Treatment of Lafora Disease
The treatment of Lafora disease is primarily focused on the limitation and severity of seizures. Medications such as valproic acid, perampanel (AMPA receptor antagonist), levetiracetam, and zonisamide are used to treat the seizures associated with Lafora disease with valproic acid being the mainstay in therapeutic intervention.
A typical ketogenic diet results in a switch to ketone body utilization by the brain as opposed to glucose which is believed to reduce neuronal glucose availability for glycogen synthesis. For this reason it is suspected that a ketogenic diet could provide some relief to Lafora disease patients. However, evidence to date, albeit in a limited number of patients, has not proven to demonstrate efficacy.