Last Updated: September 15, 2022
Introduction to CPT1 Deficiency
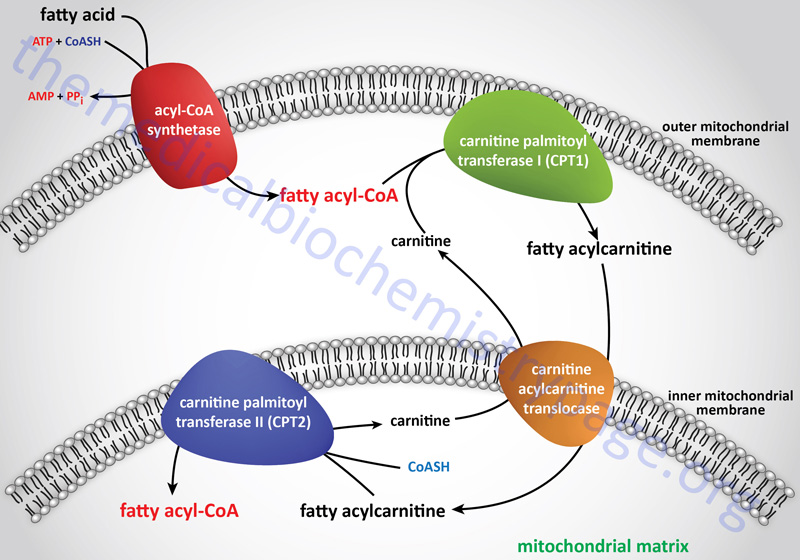
In order for the utilization of long-chain fatty acids as an energy source they must be transported into the mitochondria where the majority of fatty acid β-oxidation takes place. The transport of activated long-chain fatty acids (CoA esterified fatty acids: fatty acyl-CoAs) into the mitochondria is accomplished via a fatty acyl-carnitine intermediate, which itself is generated by the action of carnitine palmitoyltransferase 1 (CPT1 or CPT-I).
Carnitine palmitoyltransferase 1 (CPT1 or CPT-I) is one of a family of carnitine acyltransferases in humans that catalyze the reversible transfer of acyl groups between coenzyme A (CoASH) and L-carnitine, converting fatty acyl-CoA esters into fatty acyl-carnitine esters. Specifically CPT1, which is localized to the outer mitochondrial membrane, exchanges carnitine onto long-chain acyl-CoA esters, releasing free CoASH, and generating a fatty acyl-carnitine.
Molecular Biology of CPT1 Deficiency
There are three CPT1 genes in humans identified as CPT1A, CPT1B, and CPT1C. Expression of CPT1A predominates in the liver and is thus, referred to as the liver isoform. CPT1B expression predominates in skeletal muscle and is thus, referred to as the muscle isoform. CPT1C expression is exclusive to the brain and testes.
The CPT1A gene is located on chromosome 11q13.3 and consists of 22 exons that generate two alternatively spliced mRNAs encoding isoform 1 (773 amino acids) and isoform 2 (756 amino acids). CPT1 deficiency results from mutations in the CPT1A gene and as such is also referred to as CPT1A deficiency.
The CPT1B gene is located on chromosome 22q13.33 and consists of 21 exons that generate six alternatively spliced mRNAs. Five of these CPT1B mRNAs encode the same 772 amino acid protein (isoform a). The other CPT1B mRNA encodes a protein of 738 amino acids (isoform c).
The CPT1C gene is located on chromosome 19q13.33 and consists of 24 exons that generate eleven alternatively spliced mRNAs that collectively encode five distinct protein isoforms.
In addition to the CPT1 isoforms, humans express three other carnitine acyltransferases including carnitine palmitoyltransferase 2 (CPT2 or CPT-II), carnitine O-acetyltransferase (CRAT), and carnitine octanoyltransferase (CROT).
The activity of CPT1C is distinct from those of CPT1A and CPT1B in that it does not act on the same types of fatty acyl-CoAs that are substrates for the latter two enzymes, nor does it participate in the mobilization of fatty acids into the mitochondria. CPT1C does exhibit high-affinity for malonyl-CoA binding. Within the hypothalamus, brain CPT1C serves as a sensor of nutrient availability by binding malonyl-CoA which triggers a reduction in the release of the appetite promoting neuropeptides, neuropeptide Y (NPY) and Agouti-related peptide (AgRP) and an increase in the release of the appetite suppressing neuropeptides, α-melanocyte stimulating hormone (α-MSH) and cocaine and amphetamine regulated transcript (CART). The net effect of increased hypothalamic malonyl-CoA binding to CPT1C is, therefore, satiety and appetite suppression.
Mutations in the CPT1A gene, that have been associated with CPT1 deficiency, include missense and nonsense mutations, small intragenic deletions and insertions, and splice site mutations. Deletions of exons or the entire CPT1A gene as well as gene duplications have not been found in CPT1 deficiency patients.
Some populations harbor common pathogenic mutations such as the Hutterite population where mutation of the Gly to Glu at amino acid position 710 (G710E) is very common. Indeed, the frequency of carriers for this mutation in the Hutterite population is as high as 1 in 16. The Inuit populations of Greenland, Alaska, and northern Canada harbor a frequent mutation that alters the Pro at amino acid position 479 to a Leu (P479L). The frequency of CPT1 deficiency in the Inuit population is 1 in 1,000. In the general population CPT-1 deficiency is quite rare with an incidence rate of 1 in 500,000 to 1 in 1,000,000.
Clinical Features of CPT1 Deficiency
Clinical symptoms of CPT1 deficiency usually occur in an individual with a concurrent febrile or gastrointestinal illness. These circumstances result in an increase in the demand for energy and so fatty acid oxidation rates increase. In CPT1 deficient individuals the illness that precipitates the symptoms of the deficiency can be a relatively common infectious disease. However, in CPT1 deficiency the onset of symptoms is most often quite rapid and indicative of the possibility of a fatty acid oxidation defect. Indeed, hypoketotic hypoglycemia should always raise suspicion for of a disorder of fatty acid oxidation or the carnitine cycle, including CPT1 (CPT1A) deficiency.
The characteristic phenotypes observed in infants of mothers manifesting acute fatty liver of pregnancy. In children the symptoms include hepatic encephalopathy presenting with hypoketotic hypoglycemia and sudden onset of liver failure. CPT1 deficient individuals with hepatic encephalopathy will typically present with blood work demonstrating hypoglycemia and no or very low ketones, and elevated concentrations of liver transaminases (AST and ALT), ammonia, and total carnitine. CPT1 deficient patients appear developmentally and cognitively normal when not manifesting episodes of hepatic encephalopathy. If previous episodes of metabolic decompensation have resulted in neurologic damage then cognitive functions will obviously be impaired.
The CPT1A gene is expressed at the highest levels in the small intestine, kidney, and liver. These same tissues are the only gluconeogenic tissues in the body and as such have increased demands for energy during the process of endogenous glucose synthesis, particularly so in the live which is the primary (80%) producer of endogenous glucose. Due to its high level expression in the liver, CPT1 deficiency is clinically very closely related to other fatty acid metabolism and ketogenesis disorders that exhibit predominant pathology in the liver. These other disorders include MCAD deficiency, HMG-CoA synthase deficiency, and HMG-CoA lyase deficiency.
In the absence of any significant muscle or cardiac manifestations, the acute hepatic presentation of CPT1 deficiency cannot be distinguished on a clinical basis from other defects of long-chain fatty acid oxidation and conditions that present as a Reye-like illness. These other disorders include VLCAD deficiency, CPT2 deficiency, and carnitine acylcarnitine translocase (CACT) deficiency, mitochondrial trifunctional protein (MTP) deficiency (this includes the primary defect in MTP due to mutations in the long-chain 3-hydroxyacyl-CoA dehydrogenase subunit encoding gene: HADHA), and urea cycle disorders. A diagnosis of CPT1 deficiency is established by either diminished CPT1A enzyme activity or by molecular analysis of the CPT1A gene or a combination of both. CPT1A enzyme activity can be tested for in cultured skin fibroblasts. In most CPT1 deficiency patients the residual CPT1A enzyme activity is 1%-5% of normal.
Treatment of CPT1 Deficiency
Treatment of CPT1 deficiency includes a prompt response to the hypoglycemia through the administration of intravenous fluid containing 10% dextrose. Clinical protocols indicate that glucose infusion should continue in CPT1 deficient individuals past the time that it takes to normalize blood glucose concentration. The biochemical basis for continued glucose infusion is to ensure that hepatic glycogen stores are replenished.
Afflicted infants should feed frequently during the day and have cornstarch continuously at night so as to prevent hypoglycemia. In all CPT1 deficient individuals fasting should not last more than 12 hours during any illness or prior to any medical or surgical procedure. Adult CPT1 deficient individuals require a high-carbohydrate, low-fat diet to provide a constant supply of carbohydrate energy.
In addition, at least one third of total caloric intake should be from medium-chain triglycerides (MCT) as these fatty acids do not require the carnitine pathway to enter the mitochondria for oxidation. In all CPT1 deficient patients the goal of preventing hypoglycemia is to ensure that the risk for related neurologic damage is mitigated. Individuals with CPT1 deficiency should have testing of liver enzymes (AST, ALT, alkaline phosphatase) and liver function (including PT and PTT) at routine periods even when asymptomatic. The testing for liver function is even more critical in CPT1 deficient patients during periods of reduced caloric intake especially as is common during febrile illnesses.