Last Updated: September 5, 2024
Introduction to Pyruvate Carboxylase Function
Pyruvate carboxylase serves two primary functions, both of which relate to biochemical synthesis pathways. The most important functions for pyruvate carboxylase relate to these biosynthetic pathways in the liver. Pyruvate carboxylase can be considered the rate limiting enzyme in hepatic gluconeogenesis where its activation in response to accumulating acetyl-CoA is necessary to the hormonal stimulation of endogenous glucose synthesis. Despite this, the gluconeogenic enzyme, fructose-1,6-bisphosphatase is classically defined as the rate-limiting enzyme of gluconeogenesis.
The second major biosynthetic pathway involving pyruvate carboxylase is the de novo fatty acid biosynthesis pathway which is critical in the liver as a means for insulin to direct the diversion of excess glucose carbon atoms into endogenous fatty acid synthesis.
The role of pyruvate carboxylase in endogenous fatty acid synthesis is also important in other cells, particularly in neural tissues such as myelin. In the synthesis of fatty acids, the pyruvate carboxylase mediated synthesis of oxaloacetic acid from the pyruvate derived from glycolysis, ensures that accumulating mitochondrial acetyl-CoA can be converted to citrate which is then transported out of the mitochondria for delivery of mitochondrial acetyl-CoA into the cytosol. Indeed, it is the involvement of pyruvate carboxylase in driving de novo lipid synthesis in oligodendrocytes, which are the cells that synthesize myelin, that explains most of the neurological complications in pyruvate carboxylase deficiency.
The process of myelination occurs at the fastest rate in infancy which corresponds with the development of cognitive and motor skills, including language comprehension, speech acquisition, crawling and walking. Despite the significance of pyruvate carboxylase to the process of myelination, its role in hepatic gluconeogenesis is the most important reaction involving this enzyme.
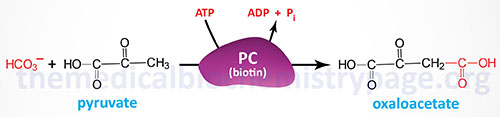
Pyruvate carboxylase is a biotin-requiring enzyme that is referred to as an ABC enzyme. The ABC acronym is derived from the role of ATP, biotin, and CO2 in its catalytic activities. Pyruvate carboxylase is a somewhat unique enzyme in that it is essentially inactive in the absence of its allosteric activator, acetyl-CoA. The primary sources of the acetyl-CoA required by pyruvate carboxylase comes from the oxidation of fatty acids which are being delivered to the liver after release from adipose tissue in response to fasting or stress.
Pyruvate carboxylase is a multi-functional enzyme and contains three distinct enzymatic domains: the biotin carboxylase (BC) domain, the carboxyltransferase (CT) domain, and the biotin carboxyl carrier protein (BCCP) domain. Functional pyruvate carboxylase is composed of four identical subunits generating an α4 homotetrameric enzyme.
The reaction catalyzed by pyruvate carboxylase occurs in a two-step process. The first partial reaction involves the fixation of CO2 to biotin that involves the BC and BCCP domains. During this initial stage of the reaction, biotin is moved to interact with the BC domain forming carboxybiotin. The carboxybiotin is brought into contact with the carboxyltransferase domain resulting in the formation of carboxylated biotin. This biotin carboxylase reaction involves a carboxyphosphate intermediate formed directly from ATP and bicarbonate. During the second step of the overall pyruvate carboxylase reaction, carboxybiotin is decarboxylated and pyruvate is concurrently carboxylated forming oxaloacetate.
Molecular Biology of Pyruvate Carboxylase
Pyruvate carboxylase is encoded by the PC gene. The PC gene is located on chromosome 11q13.2 and contains 31 exons that generate three alternatively spliced mRNAs, each of which encode the same 1178 amino acid precursor protein.
Mutations in the PC gene are the causes of pyruvate carboxylase deficiency. Pyruvate carboxylase deficiency is a rare autosomal recessive disease with an incidence rate of around 1 in 250,000 live births. The principal pathology of pyruvate carboxylase deficiency includes metabolic acidosis due to elevated lactate, failure to thrive, developmental delay, and recurrent seizures. Mutations in the PC gene that cause pyruvate carboxylase deficiency include missense mutations, deletions, and splice site mutations.
Clinical and Biochemical Features of Pyruvate Carboxylase Deficiency
Mutations in the PC gene results in three types of pyruvate carboxylase deficiency syndromes. The type A form is the infantile form and most afflicted individuals will die in infancy or early childhood. The type B form is the severe neonatal form and afflicted patients will die before three months of age. The type C form is referred to as the intermittent or benign form. Individuals with the type C form of pyruvate carboxylase deficiency experience either normal or delayed neurological development and episodic metabolic acidosis.
The lack of pyruvate carboxylase activity leads to the accumulation of pyruvate in the blood, which is subsequently converted to lactate by the enzyme lactate dehydrogenase, causing an elevated plasma concentration of lactic acid and the attendant metabolic acidosis. Alanine levels in the blood will also rise due to the cells attempts to handle the excess pyruvate through the action of alanine transaminase, ALT.
Other amino acids that are found elevated in the blood of pyruvate carboxylase deficient patients are lysine and citrulline. Citrulline is an intermediate in the urea cycle. The urea cycle is impaired in pyruvate carboxylase deficient patients due to reductions in the ability to generate sufficient amounts of the TCA cycle intermediate, 2-oxoglutarate (α-ketoglutarate). 2-Oxoglutarate can be converted to glutamate and glutamate can be used to transaminate 2-oxoglutarate, via aspartate transaminase (AST), generating aspartate. Aspartate is a required intermediate in the urea cycle. The consequences of reduced urea cycle activity are hyperammonemia which is also typical in pyruvate carboxylase deficient patients.
Type A Pyruvate Carboxylase Deficiency (PCD)
Type A PCD is an infantile onset disease characterized by mild metabolic acidosis, delayed motor development, intellectual disability, failure to thrive, hypotonia, ataxia, ocular disorder (nystagmus), and convulsions. These infants will experience episodes of metabolic acidosis, acute vomiting, and tachypnea as a result of metabolic stress or as a result of infections. Most type A PCD patients will die in infancy or early childhood, although some may survive to maturity. The most common biochemical findings in type A PCD are infantile-onset mild to moderate lactic acidosis despite the fact that measurement of the lactate to pyruvate ratio is normal in these infants. All of the type A PCD patients that have been tested were found to be harboring missense mutations in the PC gene.
Type B Pyruvate Carboxylase Deficiency (PCD)
Type B PCD infants present with hepatomegaly, convulsions, stupor, hypotonia, pyramidal tract signs (spasticity, weakness, slowing of rapid alternating movements, hyperreflexia, and a Babinski sign), and abnormal ocular behavior (nystagmus). Characteristic biochemical abnormalities in type B PCD infants include lactic acidosis, hypoglycemia, hyperammonemia, hypernatremia, and elevated concentrations of serum citrulline, proline, and lysine. However, type B PCD infants will have decreased levels of serum glutamine and the ratio of β-hydroxybutyrate to acetoacetate (ketone bodies) will also be decreased. Motor development in type B PCD infants is severely retarded and affected individuals have intellectual disability. The majority of type B PCD infants die within the first three months of life. Type B PCD patients have been found to be homozygous or to be compound heterozygotes for either missense of splice site mutations or deletions in the PC gene.
Type C Pyruvate Carboxylase Deficiency (PCD)
Type C PCD is characterized by normal or mildly delayed neurologic development. Type C PCD patients usually only experience episodic metabolic acidosis due to increases in lactate. Similar to type B PCD patients, type C PCD patients will have elevated serum proline and lysine levels. The few type C PCD patients that have been characterized were found to be compound heterozygotes.
Treatment Options for Pyruvate Carboxylase Deficiency
Treatment of the acute manifestations of pyruvate carboxylase deficiency includes intravenous glucose, hydration, and correction of the metabolic acidosis. In addition dietary supplementation with citrate, aspartic acid, and biotin have been shown to improve somatic pathologies but have not demonstrated any efficacy in the treatment of the neurological manifestations. The course of action in some patients may include liver transplantation.
Dietary inclusion of triglycerides with odd-chain fatty acids has also been shown to be beneficial in some pyruvate carboxylase deficient patients. The artificially produced triglyceride is called triheptanoin and it contains three seven-carbon saturated fatty acids. The released odd chain fatty acids provide anaplerotic substrates, in the form of succinyl-CoA, for the TCA cycle. This form of treatment is referred to as the anaplerotic diet and is also used in the treatment of very long-chain acyl-CoA dehydrogenase deficiency (VLCADD) and carnitine palmitoyltransferase 2 deficiency (CPT2D). The use of triheptanoin in pyruvate carboxylase deficient patients has shown some benefit with respect to the neurologic manifestations.
Parents should make every effort to respond appropriately and immediately and signs of decompensation in their child with pyruvate carboxylase deficiency. In addition efforts should be taken to lessen the risk for infections and environmental stressors. Pyruvate carboxylase patients should consume a diet high in carbohydrate and protein and should be fed frequently especially in the neonatal period. Periods of fasting should be avoided so as to prevent a dependence on gluconeogenesis. In addition pyruvate carboxylase deficient patients should avoid a ketogenic (high fat) diet. Pyruvate carboxylase patients should be routinely tested for levels of serum lactate.