Last Update: October 23, 2025
Introduction to Kidney Tubule Anatomy
The kidneys, in large part via the actions of numerous renal transporters, play critical roles in the regulation of numerous biochemical processes well beyond the simple excretion of metabolic waste products. One of the most important functions of the kidneys is to regulate water and electrolyte balances which ensures homeostatic composition of body fluids allowing for normal functioning of cells. In addition to water and electrolyte balance the kidneys regulate acid-base balance, regulate arterial pressures, control body fluid osmolarity, and allow for excretion of metabolic waste products and xenobiotic substances. The kidneys are also involved in the synthesis of numerous hormones including peptide hormones such as erythropoietin (EPO), several steroid hormones, and catecholamine hormones. In addition to synthesis of hormones the kidneys also function in the regulation of the production of other hormones such as calcitriol (1,25-dihydroxyvitamin D3) and angiotensin II. The synthesis of angiotensin II is regulated by the kidney through the synthesis and release of the hormone/enzyme renin. Renin is produced by a specialized smooth muscle cell present in the walls of the afferent arterioles within the glomerulus. These renin secreting cells are called juxtaglomerular cells, JG cells (also called granular cells).
The kidneys are also critical in metabolic and energy regulation through their ability to contribute to endogenous glucose production via gluconeogenesis. In order to accomplish these critical functions the kidneys are anatomically organized into substructures that are designed to allow for highly efficient filtration of components in the blood and to reabsorb components from the future urine.
Within the context of kidney anatomy there are two major organizational domains called the cortex and the medulla. The cortex represents the outer region and the medulla the inner region of each kidney. Several cell types within the cortex are responsible for the synthesis of the glucocorticoids, the mineralocorticoids, and the androgenic steroid hormones. The renal medulla is divided into cone shaped domains, 8-10 in each kidney, that are called the renal pyramids. Within the medulla there are specialized cells, medullary chromaffin cells, that synthesize the catecholamines, epinephrine and norepinephrine. The adrenal medullary chromaffin cells are functionally equivalent to postganglionic neurons of the sympathetic nervous system and in response to acetylcholine release from sympathetic preganglionic neurons these cells release epinephrine and norepinephrine into the circulation. The renal pyramids of the medulla terminate in the papilla which project into a funnel-shaped domain called the renal pelvis. The renal pelvis consists of minor and major calyces that collect urine from each of the papilla.
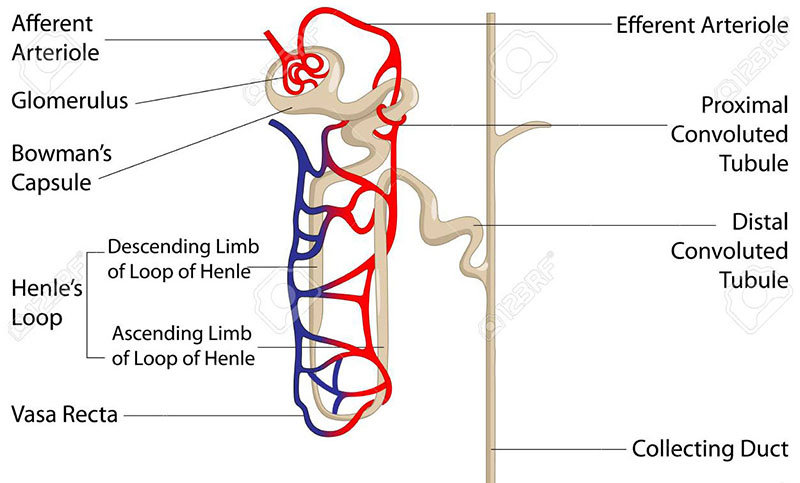
Within the renal pyramids, spanning across the outer border between the cortex and the medulla, are the blood filtering components of the kidney. These anatomical structures are called nephrons and each human kidney contains from 800,000 to 1,000,000 nephrons. Blood flow into and out of each nephron involves the arcuate artery and arcuate vein. Each nephron initiates at a compact cluster (tuft) called the glomerulus that is formed from the afferent arterioles that branch off of the arcuate artery. Each tuft of afferent arteriole is covered by epithelial cells which encase the glomerulus in what is called Bowman’s capsule. Blood flowing through the glomerular arteries is filtered by the epithelial cells that form Bowman’s capsule. The vasculature that exits from Bowman’s capsule is called the efferent arteriole which becomes the peritubular capillaries and then on the venous side becomes the renal vein.
The filtrate from Bowman’s capsule then flows through the complex structurally and functionally distinct regions of the nephron commonly referred to in its entirety as the renal tubule or the tubular filtration system. The renal tubule is composed of at least 14 segments which contain at least 16 distinct epithelial cell types. From the glomerulus, the renal tubule anatomy is structurally and functionally characterized by the proximal convoluted tubule (PCT), the proximal straight tubule (PST), the descending thin limb (DTL) of the loop of Henle, the ascending thin limb (ATL) of the loop of Henle, the thick ascending limb (TAL) of the loop of Henle, the macula densa (MD) , the distal convoluted tubule (DCT), the connecting tubule (CNT), the cortical collecting ducts, the outer medullary collecting duct, and the inner medullary collecting duct. Each of these renal tubule regions consist of specialized epithelial cells whose transport functions are outlined in the sections that follow.
Following the epithelial tubular structure of Bowman’s capsule, the filtrate enters the proximal tubule which resides within the cortical tissue of the kidney. The proximal tubule is anatomically divided into the proximal convoluted tubule (PCT; also known as pars convoluta) and the proximal straight tubule (PST; also known as pars recta). Additional complexity of the proximal tubule results from the ultrastructural organization of the cells along its length which are designated as the S1, S2, and S3 segments with S1 being next to the glomerulus and S3 farthest from the glomerulus and often being associated with the small thick portion at the beginning of the descending limb of the loop of Henle. The cellular complexity of the proximal tubule also differs, being highest in the S1 segment and lowest in the S3 segment.
Following the proximal tubule the filtrate flows into the loop of Henle which penetrates into the renal medullary tissue. The loop of Henle consists of a descending and an ascending limb. Both limbs of the loop of Henle have thick and thin regions where the thick segment of the descending limb is small in comparison to the thin segment. The thin and thick segments of the ascending limb are approximately equivalent in length.
Following the loop of Henle the nephron re-enters the renal cortical tissue where a short segment, that contains a cluster of specialized epithelial cells, forms what is called the macula densa. After leaving the macula densa the filtered fluid enters the tubular structure called the distal convoluted tubule (DCT). The DCT is followed by the connecting tubule and then the cortical collecting tubule. Along the cortical collecting tubule are several cortical collecting ducts that connect numerous nephrons together within a renal pyramid. The cortical collecting ducts combine as the tubule re-enters the renal medullary tissue and at this point the tubule is referred to as the medullary collecting tubule. The medullary collecting tubule becomes the medullary collecting ducts which merge to form progressively larger ducts that eventually empty the resulting urine into the renal pelvis through the renal papillae. Approximately 3,000-4,000 individual nephrons eventually combine together in one of these large medullary collecting ducts.
The various epithelial cells along the length of the renal tubular filtration systems have distinct functions that are dictated by the unique organization of their basolateral and apical membranes. The cells of the glomerulus are the podocytes, parietal epithelial cells, mesangial cells, and glomerular endothelial cells. Proximal convoluted tubule epithelial cells are termed PCT cells and proximal straight tubules epithelial cells are PST cells. The descending thin limb of the loop of Henle contains three epithelial cells types identified as DTL1, DTL2, and DTL3. The epithelial cells of the ascending thin limb of the loop of Henle are termed ATL cells. The thick ascending limb of the loop of Henle contains medullary (MTAL) and cortical (CTAL) epithelial cells. The division of the distal convoluted tubule is defined by DCT1 and DCT2 cells. The connecting tubule contains two distinct types of epithelial cell termed type B intercalated cells and the non-A, non-B intercalated cells. The cortical collecting duct contains three types of epithelial cell termed principal cells, type A intercalated cells, and type B intercalated cells. The outer medullary collecting duct epithelial cells include principal cells and type A intercalated cells.
The basolateral membranes of the renal tubular epithelial cells are in contact with the blood of the renal capillaries and as such can absorb electrolytes and/or compounds from the blood into the cell through the actions of specific transporter proteins and transporter complexes. These transporters are referred to as basolateral uptake transporters. In addition, the basolateral membranes can efflux electrolytes and compounds back into the blood through the actions of different transporter proteins and complexes. These transporters are referred to as basolateral efflux transporters. Similarly, the apical membranes of renal epithelial cells can efflux (apical efflux transporters) electrolytes or compounds from the cell into the renal filtrate (future urinary fluid) or reabsorb (apical uptake transporters) electrolytes or compounds from the renal filtrate through the actions of various transporter proteins or transporter complexes. Renal epithelial cells express a large number of different genes encoding the various types of basolateral and apical membrane transporter proteins and complexes.
Overview of Renal Filtration and Transport Systems
Bowman’s Capsule and Glomerulus
As blood enters the capillaries that form the tuft of the glomerulus of a nephron it is filtered into the epithelial cells that form Bowman’s capsule. Although the filtration capacity within the glomerulus is quite high, on the order of 180 liters of vascular fluid per day between both kidneys, the vast majority of that filtrate is reabsorbed as the fluid progresses along the nephrons.
The capillaries of the glomerulus are relatively impermeable to proteins and constituents of cells such that the composition of the glomerular filtrate is similar to that of the plasma. The exceptions to this are plasma constituents that are bound to protein and thus, not filtered into the cells of Bowman’s capsule. Although most plasma electrolytes are readily filtered in the glomerulus, almost half of the calcium in the plasma is bound to protein and, therefore, not filtered.
The rate of filtration of the blood that occurs within the glomerulus is called the glomerular filtration rate, GFR. The GFR is approximately 20% of renal blood flow in a healthy kidney. Overall GFR is determined by the capillary filtration coefficient and the balance of hydrostatic and osmotic forces being exerted across the capillary membrane. The high filtration capacity of glomerular capillaries is primarily due to the fact that the endothelial cell lining of these vessels is punctuated by thousands of fenestrae (holes) similar to the capillaries of the liver vasculature.
Tubular Reabsorption and Secretion
The glomerular filtrate exits Bowman’s capsule to begin its journey through the specialized tubule anatomy of the nephron. All along the nephron from the proximal tubule to the papillary ducts the composition of the original glomerular filtrate changes due to the processes of efflux and reabsorption. Although, as discussed in more detail below, there are numerous highly specialized transporters distributed in the membranes of the various different tubular epithelial cells, the generalities are outline briefly here.
As indicated above, the filtrate enters the proximal tubule where a large portion is reabsorbed which includes water, ions (e.g. Na+, Cl–, Ca2+, HCO3–, K+, and phosphate), and all organic nutrients, particularly glucose and amino acids. Secretion of organic acids and bases and hydrogen ion (H+) also occurs within the proximal tubule.
The filtrate then enters the loop of Henle, first at the descending limb and then the ascending limb. During transit down the descending limb more water is reabsorbed and up the ascending limb ions (e.g. Na+, Cl–, K+, Ca2+, Mg2+, and HCO3–) and other solutes are reabsorbed while H+ is excreted.
Within the next segment, the distal convoluted tubule (DCT), ions, acids, xenobiotics such as drugs and toxins are effluxed into the tubular lumen while some more water is reabsorbed. Within the distal convoluted tubule Na+, Cl–, Ca2+, and phosphate reabsorption occurs under the influence of hormones such as aldosterone, parathyroid hormone, and calcitriol. Sodium and chloride reabsorption within the DCT is also controlled by a family of kinases called the WNK kinases.
Within the DCT and the cortical collecting tubules there are specialized cells called principal cells and intercalated cells. There are two types of intercalated cells identified as type A (alpha) and type B (beta). Principal cells reabsorb Na+ and excrete K+. Principal cells are the major cell types that respond to the actions of aldosterone. Type A intercalated cells reabsorb K+ and HCO3– and excrete H+. Type B intercalated cells function in opposition to type A cells such that they secrete HCO3– and reabsorb H+. The differences in function between type A and type B intercalated cells is due, in part, to the opposing distributions of various transporter proteins which, for example, places a particular transporter in the apical membrane of a type A cell and the same transporter in the basolateral membrane of a type B cell.
Within the medullary collecting duct Na+, Cl–, HCO3–, and urea are reabsorbed while H+ is excreted. Water reabsorption in the medullary collecting ducts is controlled by vasopressin (anti-diuretic hormone).
Introduction to Transporter Systems of the Nephron
Proteins and protein complexes that are involved in the transport of ions and solutes across renal tubule epithelial cell membranes have an historical naming system and conventional naming system. Many of the transporters of renal tubules are not unique to the kidney but are also prominently expressed in intestinal epithelial cells and in the liver, and in many cases at some level in several other tissues as well. Renal epithelial cell transporters are also expressed on specific sides of the plasma membranes of these cells such that certain transporters are only found on the blood side membrane (basolateral) while others are found only on the luminal side membrane (apical).
Most often the functional nomenclature for renal transporters can be a bit confusing as well since a common theme is to indicate a transporter present on the basolateral (blood side) membrane is functioning as secretion transporter while a transporter found in the apical (luminal side) membrane is functioning as an uptake or reabsorption transporter. More specifically the terms uptake transporter and efflux transporter are best used to describe the functions of membrane transporters particularly in the context of renal tubular transport systems. For instance, often times a renal transporter is referred to as being involved in secretion of an ion or solute if it is found in the basolateral membrane when in fact its function may simply be the uptake, from the blood, into the cell of the ion or solute.
Once inside the cell the ion can be effluxed back into the blood or it can be effluxed across the apical membrane into the tubular lumen. Therefore, it is important to be aware that there are uptake transporters (removing substances from the blood) and efflux transporters (returning substance to the blood) present in the epithelial cell basolateral membrane. Similarly, a renal transporter is often referred to as a reabsorption transporter if it is expressed in the apical membrane, however, there are both uptake (reabsorption into the cell) and efflux (secretion into the tubular lumen) transporters in the apical membrane as in the basolateral membrane.
The majority of renal transporters are members of the solute carrier (SLC) family of transporters and the ATP binding cassette (ABC) family of transporters. The following Tables list the majority of the transporters found in the human nephron separated by domains beginning with the proximal tubule. Those transporters that are localized to the basolateral membranes and, therefore, are involved in the uptake of ions or solutes from the blood or for effluxing ions or solutes back to the blood are listed first. Those transporters that are localized to the apical membranes and, therefore, are involved in the reabsorption of ions or solutes from the lumen of the tubule or for effluxing ions or solutes to the lumen for urinary excretion are listed next.
The SLC family transporters in each membrane are listed first for each membrane followed by the ABC family transporters. Additional proteins important in renal functioning are not classically defined as transporters but are designated as channels such as the aquaporins (AQP: discussed below), the transient receptor potential (TRP) cation channels, and other various ion channels that include the calcium, chloride, and potassium channels.
It is important to note that much of the literature on renal transport systems reflects research on, and characterization of, transporters in rodent systems and there are certain species specific differences in transporter expression. As an example, the organic cation transporters encoded by the SLC22A1 (OCT1) gene and the SLC22A2 (OCT2) gene are both expressed in the basolateral membranes of the mouse proximal tubule epithelial cell. However, only the SLC22A2 (OCT2) gene encoded transporter is expressed in the human proximal tubule epithelial cell basolateral membrane.
Within each of the next sections there is a brief description of the major function of each nephron segment and a Table that includes an identification and description of transporters/channels present in that segment. The Tables are divided into four sections, two for basolateral transporters/channels (uptake from and efflux to blood) and two for apical membrane transporters/channels (reabsorption from and efflux to the lumen). Although the intention is to be comprehensive in presentation, there is likely to be omissions in the Tables.
Renal Water Balance
The renal channels responsible for water balance are members of the aquaporin (AQP) family of channels. Water transport through aquaporin channels occurs passively meaning no electrochemical gradient or energy (e.g. ATP) is required. The aquaporins are distributed throughout the nephron with some being expressed in both basolateral and apical membranes while others are expressed on intracellular membranes. Within the proximal tubule the AQP1, AQP7, AQP8, and AQP11 genes are expressed. The AQP1 protein is expressed on both the basolateral and apical membranes where its locations allow it to reabsorb and efflux water. Within the proximal tubule AQP1 is the primary aquaporin responsible for constitutive water reabsorption. The AQP1 gene is also expressed in the thin descending limb of the loop of Henle. The AQP7 channel is localized to the apical membrane of proximal tubule epithelial cells.
Several aquaporins are expressed in the connecting tubule (CNT) and collecting ducts, both cortical and medullary. The AQP2, AQP3, AQP4, AQP5, AQP6, and AQP8 genes are expressed in the CNT and cortical collecting ducts. Within the CNT and cortical collecting ducts AQP2, present in principal cells, is the primary aquaporin responsible for vasopressin-mediated regulation of fluid balance within the kidneys. In the absence of hormonal stimulation the AQP2 protein is stored within vesicles inside the cell. In response to vasopressin stimulation these vesicles fuse with the apical membrane of the principal cell and mediate rapid water reabsorption resulting in urine concentration. Mutations in the AQP2 gene are associated with a form of nephrogenic diabetes insipidus.
The AQP3 and AQP4 genes are also expressed in principal cells where their encoded proteins are localized to the basolateral membrane where they efflux water back to the blood. The AQP3 protein can facilitate movement of both water and glycerol across the membrane and as such is referred to as an aquaglyceroporin. The AQP6 gene is expressed in type A intercalated cells where it is primarily localized to intracellular vesicles. Like the AQP6 protein, AQP8 is also localized to intracellular membranes. With respect to the aquaporins, only the most significant member in each nephron segment will be included in the following Tables.
Major Renal Na+ and K+ Transporter
The major basolateral membrane transporter involved in renal Na+ and K+ exchange is the primary renal Na+/K+-ATPase which is composed of the α1-subunit encoded by the ATP1A1 gene and the β1-subunit encoded by the ATP1B1 gene. The basolateral Na+/K+-ATPase takes up two K+ ions from the blood in exchange for the efflux of three Na+ ions to the blood. The renal Na+/K+-ATPase is expressed in the basolateral membranes of epithelial cells throughout the nephron and is described here only and not listed in the following Tables.
Overview of Renal Acid-Base Balance
The kidneys play a critical role in the regulation of overall acid-base balance that ensures the maintenance of blood pH at around 7.4. The kidney accomplishes this critical function through a combination of H+, HCO3–, and NH3/NH4+ secretion and reabsorption. The proximal tubule reabsorbs approximately 80% of HCO3– present in the glomerular filtrate.
Within the proximal tubule epithelial cell apical membrane and basolateral membrane, H+ and HCO3– transporters and extracellular and intracellular carbonic anhydrases (CAIV and CAII, respectively) are involved in the processes of acid-base regulation within this segment of the nephron. Apical membrane localized NHE3 (Na+/H+-exchanger 3: encoded by the SLC9A3 gene) and V-ATPase (H+-ATPase) efflux H+ into the tubular lumen in order to titrate HCO3– present in the glomerular filtrate. At the basolateral membrane, NBC1 (Na+-bicarbonate cotransporter 1: encoded by the SLC4A4 gene) effluxes HCO3–, made de novo intracellularly via CAII, to the blood.
The proximal tubule also produces HCO3– de novo in the context of ammonia production from glutamine (discussed below). The apical membrane NHE3 and V-ATPase efflux the NH4+ into the tubule lumen and basolateral NBC1 effluxes the endogenously generated HCO3– to the blood. In the thick ascending limb (TAL), the apical membrane NHE3 and V-ATPase participate in H+ efflux and the basolateral AE2 (anion exchanger 2: encoded by the SLA4A2 gene) transporter effluxes HCO3– to the blood in exchange for Cl– uptake.
The collecting duct is also critical in the overall process of renal acid-base balance where the various specialized cell types (principal, type A intercalated, and type B intercalated) carry out net H+ exchange between the tubular lumen and the blood through the activities of V-ATPase, H+/K+-ATPase, and various Cl–/HCO3– exchangers.
The renal H+/K+-ATPases are of two types determined by different α-subunits termed the gastric (often designated HKα1) and colonic (often designated HKα2) isoforms encoded by the ATP4A and ATP12A genes, respectively. The H+/K+-ATPases also contain a β-subunit encoded by the ATP4B gene.
Within the collecting duct apical membrane localized V-ATPase, H+/K+-ATPase, and Cl–/HCO3– exchangers mediate the process of net H+ efflux to the tubular lumen. Collecting duct epithelial cell basolateral membrane Cl–-HCO3– exchangers mediate net HCO3– efflux to the blood. In type A intercalated cells of the collecting duct apical membrane, V-ATPase mediates H+ efflux to the tubular lumen. In type B intercalated cells the V-ATPase is localized to the basolateral membrane and effluxes H+ to the blood.
The transport of H+ from type A and type B intercalated cells is coordinated to the activity of various Cl–/HCO3– exchangers on the opposite membrane. In type A intercalated cells the basolateral membrane localized Cl–/HCO3– exchanger is AE1 (encoded by the SLC4A1 gene) and this transporter mediates HCO3– reabsorption from the blood. In type B intercalated cells the apical membrane localized Cl–/HCO3– exchanger is called pendrin (encoded by the SLC26A4 gene) and this transporter mediates HCO3– efflux to the tubular lumen. Both intercalated cell types also express the renal H+/K+-ATPase in the apical membrane for H+ efflux from the collecting duct to the tubular lumen.
Ammonia and the resulting ammonium ion (NH4+), like H+ and HCO3–, are central to renal acid-base balance. As discussed below, the proximal tubule is the location for endogenous ammonia production from the amino acid glutamine, a process termed renal ammoniagenesis. Approximately 50% of the newly generated ammonia produced in the proximal tubule is effluxed to the tubular lumen via the Na+/H+-exchanger 3 (NHE3; encoded by the SLC9A3 gene). The distal tubule and the collecting duct are major sites for NH4+ secretion which occurs via simultaneous efflux of H+ and NH3. The major transporters involved in this process are the Rhesus associated glycoproteins, RHBG and RHCG encoded by the RHBG (also identified as SLC42A2) and RHCG (also identified as SLC42A3) genes, respectively. The RHBG transporter is localized to the basolateral membrane of type A intercalated cells, whereas the RHCG transporter is localized to both the apical and the basolateral membranes of type A intercalated cells. Although at much lower levels RHBG and RHCG are also found in principal cells.
Renal Urea Transport and Urine Concentration
Urea represents the major nitrogen containing waste product of human metabolism. Indeed, approximately 80% of the nitrogen in the urine, on a daily basis, is in the form of urea. In addition to excretion of waste nitrogen, urea secretion is coupled to the regulation of the urine concentrating properties of the nephron, specifically within the collecting duct portion of the nephron (specifically the inner medullary collecting duct, IMCD).
Humans express two urea transporter genes, SLC14A1 and SLC14A2, that encode transporters identified as UT-B and UT-A, respectively. The SLC14A1 gene is located on chromosome 18q12.3 and is composed of 13 exons that generate six alternatively spliced mRNAs. There are two UT-B transporters, identified as UT-B1 and UT-B2, and their primary function is in the transport of urea in erythrocytes. The UT-B proteins are the basis of the Kidd blood group antigens. The expression of the UT-B1 isoform is ubiquitous being found in numerous human tissues in addition to erythrocytes. Within the kidney the UT-B1 isoform is found in the endothelial cells of the vessels (vasa recta) that are external to the collecting duct clusters. The UT-B2 isoform is only expressed in erythrocytes and the brain in humans.
The SLC14A2 gene encodes the renal urea transporters. The SLC14A2 gene is located close to the SLC14A1 gene on chromosome 18 and is composed of 23 exons. The SLC14A2 gene contains two distinct promoter elements, one that is in the typical position upstream of exon 1 and the other which resides within exon 12. The upstream promoter is termed the UT-Aα promoter and the internal promoter is termed the UT-Aβ promoter. As a result of the use of these two promoters, and the consequences of alternative splicing, at least four different UT-A transporter isoforms are generated in humans (six total are generated when considering all mammals) in distinct locations within the nephron and the rest of the body. The human UT-A isoforms that are identified as UT-A1, UT-A3, and UT-A6 are encoded by the mRNAs transcribed from the UT-Aα promoter of the human SLC14A2 gene while the UT-A2 isoform is encoded by the mRNA transcribed from the human UT-Aβ promoter. The UT-A3 protein is the N-terminal half of the UT-A1 protein while the UT-A2 protein is the C-terminal half of the UT-A1 protein. The UT-A6 protein is also common to the N-terminal region of the UT-A1 protein but is much shorter than the UT-A3 protein and the UT-A6 protein also contains unique sequences at its C-terminus.
The UT-A1 transporter is expressed in the apical membranes of epithelial cells of the inner medullary collecting duct (IMCD). The UT-A3 transporter is expressed in the basolateral membranes of the IMCD epithelial cells. However, the UT-A3 protein can be found in the apical membrane following vasopressin stimulation. The UT-A2 transporter is expressed in the basolateral membranes of epithelial cells of the thin descending limb of the loop of Henle. The UT-A1 and UT-A3 transporters are critical for urea reabsorption during antidiuresis in order to maintain the hypertonic state of the medullary interstitium (anatomically the spaces between the tubule cells and the endothelial cells of the blood vessels). In addition to renal localization, the UT-A1 isoform is expressed in the human ear (cochlea), the UT-A2 isoform is expressed in the human heart and liver, the UT-A3 isoform is expressed in the human ear (cochlea), and the UT-A6 isoform is expressed in the human colon.
The amount of urea that enters the proximal tubule from the glomerular filtration apparatus is nearly identical to the concentration in the plasma. Under normal renal physiological conditions, 30%-50% of the filtered urea is excreted. Within the proximal tubule the concentration of urea increases such that by the time the glomerular filtrate reaches the descending limb of Henle the urea concentration is 50% higher than it was in the blood. This increase in concentration results from the reabsorption of water in the proximal tubule, secondary to salt reabsorption. Urea transport in the proximal tubule is not regulated by vasopressin but it is increased as the level of Na+ and Cl– transport is increased.
The loop of Henle is permeable to urea due the presence of the UT-A2 transporter in the thin descending limb such that when the glomerular filtrate reaches the distal convoluted tubule (DCT) the concentration of urea can be on the order of 7 times that of the plasma. This level of urea concentrating occurs only under antidiuretic conditions. During water diuresis there is no proximal tubular urea transport and there is net urea reabsorption in the loop of Henle.
Within the DCT there is little urea transport such that in general the concentration decreases to approximately 70% of the load in the glomerular filtrate. Within the collecting ducts, specifically the inner medullary collecting ducts (IMCD) the actions of the UT-A1 and UT-A3 transporters result in significant urea reabsorption. The tubular urine leaving the IMCD contains approximately 50% of the filtered load of urea.
One of the principal functions of the medullary collecting ducts is urine concentration. Urea and the urea transporters in the IMCD play critical roles in the processes of urine concentration indicating that urea does not serve the sole purpose of waste nitrogen disposal. Indeed, in individuals with protein deprivation there is a significant impairment in urine concentrating and this can be restored by urea infusion (in experimental animals) or by restoration of protein intake in humans. Urea has an effective osmolarity of 0 (zero) as does water, thus, it is highly effective at being used to regulate the osmolarity of the interstitium of the nephron.
Vasopressin, which is released in response to hypothalamic osmoreceptors sensing the amount of water and Na+ in the blood, functions in the role of urea transport and urine concentration. Within the IMCD the binding of vasopressin to the V2 receptor (which is coupled to a Gs-type G-protein) triggers activation of PKA which phosphorylates the UT-A1 protein stimulating its mobilization to the apical membrane, thereby, increasing urea reabsorption as a means to maintain osmolarity under conditions of reduced water.
Proximal Tubule Transport Processes
The proximal tubule is where the vast majority of the ions and solutes in the glomerular filtrate are reabsorbed for transport back into the blood. One of the most important ions reabsorbed from the glomerular filtrate via the proximal tubule is Na+ which is primarily, although certainly not exclusively, by the ubiquitously distributed Na+,K+-ATPase described above. The proximal tubule is also a major site for the regulation of the acid-base balance of the glomerular filtrate. This latter function is effected by the efflux of H+ from the proximal tubule epithelial cells into the blood in exchange for bicarbonate ion in the glomerular filtrate.
With respect to proximal tubule function, in a general sense, roughly 70% of all the ions and water in the glomerular filtrate are reabsorbed. Proximal tubular reabsorption rates are different for the various constituents in the glomerular filtrate. For example, 100% of the organic ions, particularly glucose, are reabsorbed, approximately 60%-70% of the calcium is reabsorbed, approximately 85% of phosphate ion is reabsorbed, approximately 65% of potassium ion is reabsorbed, and approximately 50% of the urea is reabsorbed.
The proximal tubule is also the location where 99% of amino acids in the glomerular filtrate are reabsorbed. The majority of the calcium, magnesium, and phosphate that is reabsorbed by the proximal tubule is accomplished by passive paracellular transport with only 10%-15% occurring via active transport across the apical and basolateral membranes. Paracellular transport is that occurring between cells as opposed to transcellular transport which refers to transport through cells. Paracellular transport processes are regulated by tight junctions and by the electrochemical gradients established across the tubule via the actions of the numerous apical membrane-localized ion transporters.
Proximal Tubule Ammoniagenesis
Another important function of the proximal tubule is the generation of ammonia (NH3) which is in equilibrium with ammonium ion (NH4+). The production of ammonia is referred to as ammoniagenesis. Although ammonia and ammonium ion excretion in the urine constitutes a portion of the total waste nitrogen excretion, ammoniagenesis in the kidney is critically important in acid-base regulation. The production, secretion, and reabsorption of bicarbonate (HCO3–), by the kidney, is a major contributor to the renal responsibility to regulate acid-base balance. However, on its own renal bicarbonate production is not sufficient, thus the need for ammonia generation. The ionization of NH3 to NH4+ is an effective means to reduce the overall H+ load, and thus, raise the pH of the blood during periods of acidosis.
The major source of nitrogen for proximal tubule ammoniagenesis is the amino acid glutamine which can yield two moles of NH4+ through the concerted actions of glutaminase and glutamate dehydrogenase, respectively. Proximal tubule glutamine reabsorption from the glomerular filtrate is accomplished via the action of the apical membrane associated Na+-dependent neutral amino acid transporter 1 identified as B0AT1 and encoded by the SLC6A19 gene. Mutations in the SLC6A19 gene are the causes of Hartnup disorder. Glutamine is also taken up from the blood by proximal tubule cells via the basolateral membrane Na+-coupled neutral amino acid transporter 3 identified as SNAT3 which is encoded by the SLC38A3 gene. The biochemistry of ammonia release from glutamine is covered in detail in the Amino Acid Catabolism page.
In addition to releasing two moles of NH4+, the benefits of renal glutamine metabolism are that bicarbonate (HCO3–) is also generated. The HCO3– is derived from the TCA cycle-mediated oxidation of the 2-oxoglutarate that results from the glutaminase and glutamate dehydrogenase reactions. The HCO3– is then transported to the blood from the proximal tubule cells via the basolateral membrane Na+-coupled bicarbonate transporter identified as NBC1 which is encoded by the SLC4A4 gene. Mutations in the SLC4A4 gene result in type 2 (proximal) renal tubular acidosis (type 2 RTA). The HCO3– produced by the proximal tubule via glutamine metabolism represents new HCO3– being added to the pool already in the blood, thereby enhancing the buffering capacity of the blood. Approximately 50% of the ammonia/ammonium produced from glutamine in the proximal tubule is effluxed to the tubular lumen via the Na+/H+-exchanger 3 (NHE3) which is encoded by the SLC9A3 gene. The remainder of the ammonia/ammonium enters the systemic circulation via the renal veins where it is picked up by the liver and incorporated into urea. Ammonia produced by the proximal tubule and transported to the lumen can be reabsorbed in the loop of Henle and finally excreted by the collecting duct.
Table of Proximal Tubule Transporters/Channels
| Gene | Common/Other Name | Functions / Comments |
| Basolateral Membrane Uptake from Blood | ||
| SLC9A1 | NHE1: Na+/H+ exchanger 1 | uptake of Na+ with simultaneous efflux of H+ |
| SLC9A4 | NHE4: Na+/H+ exchanger 4 | uptake of Na+ with simultaneous efflux of H+ |
| SLC13A1 | NaS1: Na+/sulfate cotransporter | Na+ and sulfate uptake; major contributor to renal sulfate homeostasis |
| SLC13A3 | NaDC3: Na+-dependent carboxylate cotransporter 3 | Na+ and carboxylate uptake; citrate, succinate, 2-oxoglutarate (α-ketoglutarate) uptake |
| SLC22A2 | OCT2: organic cation transporter 2 | secretion; organic cation uptake from blood; predominant organic cation transporter in the basolateral membranes of proximal tubule cells; major creatinine uptake transporter |
| SLC22A3 | OCT3: organic cation transporter 3 | secretion; organic cation uptake from blood; major creatinine uptake transporter |
| SLC22A6 | OAT1: organic anion transporter 1 | secretion; organic anion uptake from the blood in exchange for dicarboxylic acid such as glutarate or 2-oxoglutarate (α-ketoglutarate); involved in uric acid uptake from blood; functions with SLC22A8 (OAT3) |
| SLC22A7 | OAT2: organic anion transporter 2 | secretion; organic anion uptake from the blood in exchange for dicarboxylic acid such as glutarate or 2-oxoglutarate (α-ketoglutarate); uptake of uric acid; major creatinine uptake transporter; removes numerous drugs from blood (e.g. 5-flurouracil) |
| SLC22A8 | OAT3: organic anion transporter 3 | secretion; organic anion uptake from the blood in exchange for dicarboxylic acid such as glutarate or 2-oxoglutarate (α-ketoglutarate); involved in uric acid uptake from blood; functions with SLC22A8 (OAT1); removes numerous drugs from blood including methotrexate, indomethacin, and ciprofloxacin |
| SLC38A3 | SNAT3: system N/A transporter 3 | glutamine uptake from and efflux to blood |
| SLCO4C1 | OATP4C1: organic anion transporting polypeptide 4C1 | secretion; uptake from the blood; transports bile acids, peptides, certain drugs, conjugated steroids, thyroid hormones |
| Basolateral Membrane Efflux to Blood | ||
| AQP1 | aquaporin 1 | major proximal tubule water reabsorbing channel; present in both basolateral and apical membranes for water reabsorption and efflux to the blood |
| SLC2A9 | GLUT9; URATv1 | SLC2A9 gene yields two alternatively spliced mRNAs generating variants identified as GLUT9a and GLUT9b; the two forms are asymmetrically distributed such that GLUT9a is found in the basolateral membrane while GLUT9b is found in the apical membrane; GLUT9a involved in uric acid, fructose, and glucose efflux back to blood |
| SLC3A2 | 4F2hc | constitutes the heavy chain of heteromeric amino acid transporters; associates with the light chain proteins encoded by the SLC7A5, SLC7A6, SLC7A7, SLC7A8, SLC7A10 and SLC7A11 genes; amino acid efflux to blood |
| SLC4A4 | NBC1: Na+-bicarbonate cotransporter 1 | Na+ and bicarbonate efflux to blood; mutations in SLC4A4 gene associated with type 2 (proximal) renal tubular acidosis, RTA |
| SLC7A7 | y+LAT1: y+L-type amino acid transporter 1 | constitutes the light chain of a heteromeric cationic amino acid transporter; associates with the heavy chain protein encoded by the SLC3A2 gene (protein also identified as 4F2hc); cationic amino acid efflux to blood |
| SLC7A8 | LAT2: L-type amino acid transporter 2 | constitutes the light chain of a heteromeric cationic amino acid transporter; associates with the heavy chain protein encoded by the SLC3A2 gene (protein also identified as 4F2hc); cationic amino acid efflux to blood |
| SLC9A1 | NHE1: Na+/H+ exchanger 1 | efflux of H+ with simultaneous uptake of Na+ |
| SLC9A4 | NHE4: Na+/H+ exchanger 4 | efflux of H+ with simultaneous uptake of Na+ |
| SLC16A10 | TAT1: T-type amino acid transporter 1 | Na+-independent aromatic amino acid (phenylalanine, tyrosine, and tryptophan) efflux to blood |
| SLC26A1 | SAT1: sulfate anion transporter 1 | sulfate efflux to blood; mutations in gene associated with hyperoxaluria and formation of calcium oxalate kidney stones |
| SLC38A3 | SNAT3: system N/A transporter 3 | glutamine uptake from and efflux to blood |
| SLC51A | OSTα: organic solute transporter alpha | reabsorption; functions as heterodimer with SLC51B (OSTβ); uptake of bile acids, steroids, and prostaglandin E2 (PGE2) |
| SLC51B | OSTβ: organic solute transporter beta | reabsorption; functions as a heterodimer with SLC51A (OSTα); uptake of bile acids, steroids, and prostaglandin E2 (PGE2) |
| ABCA1 | reabsorption; cholesterol and phospholipid transfer | |
| ABCC1 | MRP1: multidrug resistance associated protein 1 | reabsorption; leukotriene C4 (LTC4); glutathione conjugated xenobiotics |
| ABCC3 | MRP3: multidrug resistance associated protein 3 | reabsorption; also called MOATD (multispecific organic anion transporter D) and CMOAT2 (canalicular multispecific organic anion transporter 2) |
| ABCC5 | MRP5: multidrug resistance associated protein 5 | reabsorption; uptake of cyclic nucleotides from tubular lumen; also called MOATC (multispecific organic anion transporter C) |
| ABCC6 | MRP6: multidrug resistance associated protein 6 | reabsorption; also called MOATE (multispecific organic anion transporter E) |
| Apical Membrane Uptake from Tubular Lumen | ||
| AQP1 | aquaporin 1 | major proximal tubule water reabsorbing channel; present in both basolateral and apical membranes for water reabsorption from lumen and efflux to the blood |
| SLC1A1 | EAAT3: excitatory amino acid transporter 3 | high affinity glutamate transporter; also transports aspartate and cystine; Na+ and H+ taken up with amino acid with simultaneous K+ efflux |
| SLC2A9 | GLUT9; URATv1 | SLC2A9 gene yields two alternatively spliced mRNAs generating variants identified as GLUT9a and GLUT9b; the two forms are asymmetrically distributed such that GLUT9a is found in the basolateral membrane while GLUT9b is found in the apical membrane; GLUT9b involved in uric acid, fructose, and glucose reabsorption from tubular lumen |
| SLC3A1 | rBAT: renal basic amino acid transporter | is a heavy chain subunit of heteromeric amino acid transporters; functions with the light chain subunit encoded by the SLC7A9 gene; cystine reabsorption along with amino acid efflux; mutations in gene cause type I cystinurias |
| SLC5A1 | SGLT1: Na+-glucose transporter 1 | primarily expressed in the S2 segment; of little significance to renal glucose reabsorption |
| SLC5A2 | SGLT2: Na+-glucose transporter 2 | predominantly expressed in the S1 segment; responsible for approximately 90% of glucose reabsorption |
| SLC5A8 | SMCT1: sodium monocarboxylate transporter 1 | uric acid reabsorption along with lactate efflux; functions with SLC5A12 (SMCT2); is a member of the sodium-glucose transporter family (SGLT) |
| SLC5A12 | SMCT2: sodium monocarboxylate transporter 2 | uric acid reabsorption along with lactate efflux; functions with SLC5A8 (SMCT1); is a member of the sodium-glucose transporter family (SGLT) |
| SLC6A15 | B0AT2: B0 neutral amino acid transporter 2 | reabsorption of proline, leucine, valine, isoleucine, and methionine along with Na+ |
| SLC6A18 | B0AT3: B0 neutral amino acid transporter 3 | neutral amino acid (glycine, alanine, methionine, serine, cysteine) reabsorption along with Na+; apical membrane localization of SLC6A18 requires the protein called collectrin which is encoded by the TMEM27 gene |
| SLC6A19 | B0AT1: B0 neutral amino acid transporter 1 | neutral amino acid reabsorption along with Na+; mutations in gene cause Hartnup disorder; apical membrane localization of SLC6A19 requires the protein called collectrin which is encoded by the TMEM27 gene |
| SLC6A20 | uptake of proline, hydroxyproline, glycine, and betaine (trimethylglycine) along with Na+; apical membrane localization of SLC6A20 requires the protein called collectrin which is encoded by the TMEM27 gene | |
| SLC7A9 | b0,+AT | is a light chain subunit of heteromeric amino acid transporters; functions with the heavy chain subunit encoded by the SLC3A1 gene; cystine reabsorption along with amino acid efflux; mutations in gene cause non-type I cystinurias |
| SLC9A3 | NHE3: Na+/H+ exchanger 3 | major transporter involved in proximal tubule reabsorption of Na+ and HCO3– with simultaneous efflux of H+ or NH4+ |
| SLC9A8 | NHE8: Na+/H+ exchanger 8 | uptake of Na+ with simultaneous efflux of H+; expressed at highest levels in the neonate |
| SLC10A2 | ASBT: apical sodium bile transporter | reabsorption; H+-coupled uptake of small peptides and β-lactam (e.g. penicillin and cephalosporins) antibiotics |
| SLC13A2 | NaDC1: Na+-dependent carboxylate cotransporter 1 | Na+ and carboxylate uptake; citrate, succinate, 2-oxoglutarate (α-ketoglutarate) uptake; major regulator of renal dicarboxylate reabsorption |
| SLC15A1 | PEPT1: peptide transporter 1 | reabsorption; H+-coupled uptake of small peptides and β-lactam (e.g. penicillin and cephalosporins) antibiotics, angiotensin converting enzyme (ACE) inhibitors, several prodrugs |
| SLC15A2 | PEPT2: peptide transporter 2 | reabsorption; H+-coupled uptake of small peptides and β-lactam (e.g. penicillin and cephalosporins) antibiotics, angiotensin converting enzyme (ACE) inhibitors, several prodrugs |
| SLC20A2 | Pit-2: inorganic phosphate (Pi) transporter 2 | member of the type III Na+-phosphate cotransporter family; phosphate ion (as H2PO4–) uptake simultaneously with Na+; considered to play a minor role in phosphate reabsorption |
| SLC22A4 | OCTN1: multispecific organic cation transporter 1 | organic cation efflux in exchange for H+ uptake from lumen; reabsorption of carnitine, ergothioneine (thiourea derivative of histidine made by bacteria), and acetylcholine |
| SLC22A5 | OCTN2: multispecific organic cation transporter 2 | Na+-dependent carnitine and organic cation reabsorption |
| SLC22A7 | OAT2: organic anion transporter 2 | organic anion uptake from the lumen in exchange for dicarboxylic acid such as glutarate or 2-oxoglutarate (α-ketoglutarate); involved in creatinine reabsorption |
| SLC22A10 | OAT5: organic anion transporter 5 | organic anion uptake from the lumen in exchange for dicarboxylic acid such as glutarate or 2-oxoglutarate (α-ketoglutarate) |
| SLC22A11 | OAT4: organic anion transporter 4 | reabsorption of uric acid in exchange for carboxylate (primarily 2-oxoglutarate) efflux; also involved in transport of diuretics into the tubular lumen for excretion |
| SLC22A12 | URAT1: urate transporter 1 | reabsorption; major transporter involved in uric acid reabsorption; functions in exchange for carboxylate with primary exchange being lactate; target of uricosuric drugs (e.g. probenecid, benzobromarone); inhibition can lead to lactate retention and lactic acidosis |
| SLC22A13 | OAT10: organic anion transporter 10 | reabsorption of uric acid in exchange for monocarboxylate efflux; also involved in uptake of nicotinate |
| SLC23A1 | SVCT1: Na+-dependent vitamin C transporter 1 | reabsorption of ascorbate |
| SLC26A1 | SAT1: sulfate anion transporter 1 | sulfate and oxalate reabsorption; mutations in gene associated with hyperoxaluria and formation of calcium oxalate kidney stones |
| SLC26A6 | reabsorption of Cl– in exchange for HCO3– efflux; also involved in oxalate and sulfate anion transport | |
| SLC28A1 | CNT1: Na+-dependent concentrative nucleoside transporter 1 | reabsorption; uptake of nucleosides |
| SLC28A2 | CNT2: Na+-dependent concentrative nucleoside transporter 2 | reabsorption; uptake of nucleosides |
| SLC28A3 | CNT3: Na+-dependent concentrative nucleoside transporter 3 | reabsorption; uptake of nucleosides |
| SLC29A1 | ENT1: equilibrative nucleoside transporter 1 | reabsorption; uptake of nucleosides |
| SLC29A2 | ENT2: equilibrative nucleoside transporter 2 | reabsorption; uptake of nucleosides |
| SLC34A1 | NPT2a (NPT2): Na+-phosphate co-transporter 2a | primary transporter for uptake of phosphate ion (as HPO42–) along with Na+; transports three Na+ with each phosphate ion; functions in concert with NPT2c for phosphate reabsorption |
| SLC34A3 | NPT2c: Na+-phosphate co-transporter 2c | primary transporter for uptake of phosphate ion (as HPO42–) along with Na+; transports two Na+ with each phosphate ion; functions in concert with NPT2a for phosphate reabsorption |
| SLC36A1 | PAT1: proton amino acid transporter 1 | H+-coupled proline, glycine, and alanine uptake |
| SLC36A2 | PAT2: proton amino acid transporter 2 | H+-coupled proline, glycine, and alanine uptake |
| SLCO1A2 | OATP1A2/OATPA: organic anion transporting polypeptide A | OATP1A2 is human homolog of rodent OAT-K1/K2; formerly identified as SLC21A3; Na+-independent uptake of bile acids |
| SLCO1B1 | OATP1B1/OATPC: organic anion transporting polypeptide C | OATP1B1 is human homolog of rodent OATP1A4; formerly identified as SLC21A6; Na+-independent uptake of bilirubin, glucuronidated estradiol, leukotriene C4 (LTC4) |
| SLCO2A1 | OATP2A1 | OATP1B1 is human homolog of rodent OATP1A6; formerly identified as SLC21A2; Na+-independent uptake of prostaglandins |
| SLCO2B1 | OATP2B1/OATPB: organic anion transporting polypeptide B | formerly identified as SLC21A9; Na+-independent uptake of sulfated steroids |
| SLCO3A1 | OATP3A1/OATPD: organic anion transporting polypeptide D | formerly identified as SLC21A11; Na+-independent uptake of prostaglandins, thyroxine (T4), and vasopressin |
| SLCO4A1 | OATP4A1/OATPE: organic anion transporting polypeptide E | OATP4A1 is human homolog of rodent OATP1A1; formerly identified as SLC21A12; Na+-independent uptake of thyroid hormones and bile acids |
| TRPC1 | transient receptor potential canonical 1; Ca2+ reabsorption transporter | |
| Apical Membrane Efflux to Tubular Lumen | ||
| SLC9A3 | NHE3: Na+/H+ exchanger 3 | major transporter involved in proximal tubule reabsorption of Na+ and HCO3– with simultaneous efflux of H+ |
| SLC9A8 | NHE8: Na+/H+ exchanger 8 | uptake of Na+ with simultaneous efflux of H+; expressed at highest levels in the neonate |
| SLC17A1 | NPT1: Na+-phosphate transporter 1 | uric acid efflux; phosphate reuptake via Na+ co-transport; missense mutation converting Ile at position 269 to Thr (I269T mutation) enhances urate secretion preventing renal under excretion gout |
| SLC17A3 | NPT4: Na+-phosphate transporter 4 | uric acid efflux; loop diuretics, such as furosemide and bumetanide interact with SLC17A3 and are excreted; competition for secretion may be involved in diuretic-induced hyperuricemia |
| SLC22A4 | OCTN1: multispecific organic cation transporter 1 | organic cation efflux in exchange for H+ uptake from lumen; also functions in reabsorption (as described above) of carnitine; involved in renal efflux of gabapentin and mutations in SLC22A4 gene associated reduced secretion of the drug |
| SLC26A6 | efflux of HCO3– in exchange for Cl– reabsorption; also involved in oxalate and sulfate anion transport | |
| SLC47A1 | MATE1: multidrug and toxin extrusion protein 1 | creatinine and organic cation efflux to tubular lumen; in addition to organic cations SLC47A1 also transports the anionic steroid estrone sulfate; also transports several drugs such as metformin, cisplatin, cimetidine, topotecan, procainamide, acyclovir, and ganciclovir |
| SLC47A2 | MATE2-K: multidrug and toxin extrusion protein 2 | creatinine and organic cation efflux to tubular lumen; effluxes several drugs similar to those transported by SLC47A1 such as metformin, cimetidine, and procainamide; the antibiotic pyrimethamine is a potent inhibitor of SCL47A2 function |
| ABCB1 | MDR1: multidrug resistance protein 1 | secretion; also called P-gp or PGY1 (P-glycoprotein 1) |
| ABCC2 | MRP2: multidrug resistance associated protein 2 | secretion; efflux of non-bile organic ions; also called CMOAT (canalicular multispecific organic anion transporter) |
| ABCC4 | MRP4: multidrug resistance associated protein 4 | secretion; efflux of uric acid; also called MOATB (multispecific organic anion transporter B) |
| ABCG2 | BCRP: breast cancer resistance protein | secretion; involved in uric acid efflux; efflux of xenobiotics |
Loop of Henle
The loop of Henle gets its name from the German anatomist who first described this anatomical structure of the nephron, Friedrich Gustav Jakob Henle. The loop of Henle is composed of descending and ascending limbs which both can be divided into two broad domains termed thin and thick. The upper part of the descending limb is sometimes referred to as the thick descending limb although most often the entirety of the descending limb is described as only being thin. The ascending limb is clearly divided anatomically and functionally into thin and thick segments with the most significant transport processes occurring with the thick ascending limb (TAL).
The primary function of the loop of Henle is water, sodium, and chloride (salt) reabsorption from the glomerular filtrate. This reabsorption process in the loop of Henle results in the formation of a concentration gradient within the medullary portion of the loop. The descending limb of the loop is highly permeable to water but relatively impermeable to ions and solutes. Conversely the ascending limb of the loop is permeable to ions while being relatively impermeable to water. This difference in water and ion permeability between the descending and ascending limbs of the loop of Henle is what results in the concentration gradient to the glomerular filtrate.
Thick Ascending Limb, TAL
The thick ascending limb (TAL) of the loop of Henle is principally responsible for sodium and chloride ion reabsorption from the glomerular filtrate. Approximately 25%-30% of these ions are reabsorbed by the transporters of the epithelial cells in the TAL. The major transporter tasked with sodium and chloride reabsorption is the SLC12A1 gene encoded protein expressed in the apical membrane of the cell. The SLC12A1 encoded protein is more commonly called NKCC2 (Na+/K+/2 Cl– cotransporter 2). While reabsorbing salt the TAL reabsorbs no water which results in a dilution of the ionic content of the luminal fluid.
Absorption of sodium and chloride, via the action of NKCC2, requires that an electrochemical gradient be established. This gradient is generated via the action of the ubiquitously expressed Na+,K+-ATPases expressed in the basolateral membranes of the epithelial cells throughout the nephron as discussed earlier. The Na+ reabsorbed by NKCC2 is effluxed to the blood across the basolateral membrane by the Na+/K+-ATPase while the Cl– is effluxed to the blood via the basolateral K+/Cl– cotransporter encoded by the SLC12A7 gene. The SLC12A7 encoded protein is commonly called KCC4 (K+/Cl– cotransporter 4).
The NKCC2 reabsorbed Cl– can also be effluxed to the blood via the basolateral voltage-gated Cl– transporter encoded by the CLCNKB gene. The CLCNKB encoded protein is commonly called ClC-Kb (the mouse protein is ClC-Kb2). Some of the reabsorbed K+ is effluxed back into the lumen through the action of the KCNJ1 encoded potassium channel more commonly called ROMK (renal outer medullary potassium channel).
Within the loop of Henle approximately 20% of the Ca2+ in the glomerular filtrate is reabsorbed, all of which occurs in the TAL. Calcium reabsorption in the TAL occurs by both active transport across the cell membrane (transcellular) and by paracellular passive transport with the bulk occurring via the paracellular route. The paracellular transport of Ca2+ in the TAL is driven by the electrochemical gradient that is established across the tubule via the actions of the apical transporters ROMK and NKCC2.
Table of Thick Ascending Limb (TAL) Transporters/Channels
| Gene | Common/Other Name | Functions / Comments |
| Basolateral Membrane Uptake from Blood | ||
| SLC9A4 | NHE4: Na+/H+ exchanger 4 | uptake of Na+ with simultaneous efflux of H+; expressed throughout nephron but highest in TAL |
| TRPV4 | transient receptor potential, vanilloid 4 | calcium uptake from blood |
| Basolateral Membrane Efflux to Blood | ||
| SLC9A4 | NHE4: Na+/H+ exchanger 4 | efflux of H+ and NH4+ with simultaneous uptake of Na+; expressed throughout nephron but highest in TAL |
| SLC12A7 | KCC4: potassium-chloride cotransporter 4 | effluxes both K+ and Cl– to blood |
| CLCNKA | ClC-Ka: chloride channel Ka | voltage-gated chloride channel; exclusively expressed in the kidney and inner ear; requires an accessory β-subunit called barttin encoded by the BSND gene; minor chloride transporter of the TAL; effluxes Cl– ion to the blood that was reabsorbed from the glomerular filtrate via the actions of SLC12A1 (NKCC2) and SLC12A3 (NCC) |
| CLCNKB | ClC-Kb: chloride channel Kb | voltage-gated chloride channel; exclusively expressed in the kidney and inner ear; requires an accessory β-subunit called barttin encoded by the BSND gene; predominant chloride transporter of the TAL; effluxes Cl– ion to the blood that was reabsorbed from the glomerular filtrate via the actions of SLC12A1 (NKCC2) and SLC12A3 (NCC); mutations in gene are the cause of Bartter syndrome type 3 |
| KCNJ10 | Kir4.1 | member of the inwardly-rectifying potassium channel family; coupled to the actions of the Na+/K+-ATPase for efflux of K+ to the blood |
| KCNJ16 | Kir5.1 | member of the inwardly-rectifying potassium channel family; coupled to the actions of the Na+/K+-ATPase for efflux of K+ to the blood |
| Apical Membrane Uptake from Tubular Lumen | ||
| SLC9A2 | NHE2: Na+/H+ exchanger 2 | uptake of Na+ with simultaneous efflux of H+ |
| SLC9A3 | NHE3: Na+/H+ exchanger 3 | uptake of Na+ with simultaneous efflux of H+ |
| SLC12A1 | NKCC2: Na+/K+/2 Cl– cotransporter 2 | transports one Na+, one K+, and two Cl– ions; exclusively expressed in the TAL; responsible for all of the NaCl reabsorption occurring in the TAL; also responsible for NH4+ reabsorption; at least six isoforms result from alternative splicing and these different forms are differentially distributed along the TAL and also exhibit differences in ion transport properties; trafficking of NKCC2 in thick ascending limb cells is controlled by its state of phosphorylation; phosphorylation of NKCC2 is carried out by WNK kinases following their activation by vasopressin; NKCC2 is the target of the loop diuretics furosemide and bumetanide; mutations in gene are the cause of Bartter syndrome type 1 |
| SLC26A6 | reabsorption of Cl– in exchange for HCO3– efflux; also involved in oxalate and sulfate anion transport | |
| TRPP2 | transient receptor potential polycystin 2; Ca2+ reabsorption transporter | |
| TRPV4 | transient receptor potential vanilloid 4; Ca2+ reabsorption transporter | |
| Apical Membrane Efflux to Tubular Lumen | ||
| SLC9A2 | NHE2: sodium hydrogen (H+) exchanger 2 | efflux of H+ with simultaneous uptake of Na+ |
| SLC26A6 | efflux of HCO3– in exchange for Cl– reabsorption; also involved in oxalate and sulfate anion transport | |
| KCNJ1 | ROMK: renal outer medullary potassium channel | K+ efflux to lumen; members of the KCNJ family of inwardly rectifying voltage-gated potassium channels are also identified a Kir channels with ROMK being Kir1.1 (three isoforms due to alternative splicing: Kir1.1a, Kir1.1b, and Kir1.1c); aldosterone activates ROMK by inducing its phosphorylation by SGK1 (serum/glucocorticoid regulated kinase 1) which results in potassium secretion; mutations in gene are the cause of Bartter syndrome type 2 |
Distal Convoluted Tubule, DCT
The distal convoluted tubule (DCT) is a short segment of the nephron that represents the region between the macula densa and collecting duct. The primary function of the DCT is the regulation of extracellular fluid volume and electrolyte homeostasis. While carrying out ion transport, the epithelial cells of the DCT remain relatively impermeable to water. Cells of the DCT are responsive to the actions of the hormones angiotensin II and aldosterone with the late convoluted tubule region (denoted DCT2) representing a portion of the aldosterone-sensitive distal nephron which includes the connecting tubule (CNT) and the collecting duct.
The basolateral membranes of the epithelial cells of the DCT have the highest concentrations of the Na+/K+-ATPase of all cells along the nephron. The DCT epithelial cells reabsorb Na+ and Cl– ions across the apical membrane, primarily via the actions of the thiazide-sensitive sodium and chloride cotransporter identified as NCC (encoded by the SLC12A3 gene). Thiazides represent a class of diuretic drugs used in the treatment of hypertension and edema. The epithelial cells of the DCT are also important for magnesium reabsorption. Mutations in genes encoding transporters and channels found in the DCT are associated with several diseases in humans including Bartter syndrome, Gitelman syndrome, familial hyperkalemic hypertension, EAST syndrome (Epilepsy, Ataxia, Sensorineural deafness, and salt-wasting renal Tubulopathy), and hereditary hypomagnesemias.
The DCT contributes to overall Ca2+ reabsorption accounting for approximately 10%-15% of the total reabsorption. However, unlike in the proximal tubule and the TAL of the loop of Henle, all of the Ca2+ reabsorption in the DCT occurs via active (transcellular) transport. The active transport of Ca2+, within the DCT, is regulated by parathyroid hormone, PTH. The apical membrane associated transient receptor potential vanilloid 5 (TRPV5) transporter is the major Ca2+ uptake transporter in the DCT. The intracellular Ca2+ is then transported across the cell to the basolateral membrane via the calcium-binding protein, calbindin-D28K. Efflux of the reabsorbed Ca2+ to the blood occurs through the actions of the basolateral membrane transporters encoded by the SCL8A1 and ATP2B1 genes. The transporter encoded by the SLC8A1 gene is identified as NCX1 (Na+/Ca2+ exchanger 1). The transporter encoded by the ATP2B1 gene is identified as PMCA1b (plasma membrane Ca2+ transporter 1b).
Table of Distal Convoluted Tubule Transporters/Channels
| Gene | Common/Other Name | Functions / Comments |
| Basolateral Membrane Uptake from Blood | ||
| SLC8A1 | NCX1: Na+/Ca2+-exchanger 1 | primarily localized to the late DCT (DCT2); uptake of Na+ in exchange for efflux of Ca2+ to blood |
| SLC12A2 | NKCC1: Na+/K+/2 Cl– cotransporter 1 | primarily localized to the late DCT (DCT2); uptake of ammonia (NH3) from the blood along with Na+, K+, and Cl– |
| TRPV5 | transient receptor potential vanilloid 5; Ca2+ uptake channel; present in the late DCT2 region; can form heteromeric complexes with TRPV6; co-localizes with the calcium-binding protein calbindin-D28K | |
| Basolateral Membrane Efflux to Blood | ||
| ATP2B1 | PMCA1b: plasma membrane Ca2+ transporter 1b | ATP-hydrolysis-mediated Ca2+ efflux; expressed in the late DCT region (DCT2); the PMCA1b protein isoform is derived from a specific alternatively spliced mRNA from the ATP2B1 gene |
| SLC8A1 | NCX1: Na+/Ca2+-exchanger 1 | primarily localized to the late DCT (DCT2) and connecting tubule (CNT); effluxes Ca2+ to blood in exchange for uptake of Na+ |
| SLC12A7 | KCC4: potassium-chloride cotransporter 4 | effluxes both K+ and Cl– to blood |
| CLCNKB | ClC-Kb: chloride channel Kb | voltage-gated chloride channel; exclusively expressed in the kidney and inner ear; requires an accessory β-subunit called barttin encoded by the BSND gene; predominant chloride transporter of the TAL; effluxes Cl– ion to the blood that was reabsorbed from the glomerular filtrate via the actions of SLC12A1 (NKCC2) and SLC12A3 (NCC); mutations in gene are the cause of Bartter syndrome type 3 |
| CNNM2 | cyclin and CBS domain divalent metal cation transport mediator 2 | involved in Mg2+ efflux to blood |
| KCNJ10 | Kir4.1 | member of the inwardly-rectifying potassium channel family; coupled to the actions of the Na+,K+-ATPase for efflux of K+ to the blood |
| KCNJ16 | Kir5.1 | member of the inwardly-rectifying potassium channel family; coupled to the actions of the Na+,K+-ATPase for efflux of K+ to the blood |
| Apical Membrane Uptake from Tubular Lumen | ||
| SLC9A2 | NHE2: sodium hydrogen (H+) exchanger 2 | uptake of Na+ with simultaneous efflux of H+ |
| SLC12A3 | NCC: Na+/Cl– cotransporter | major aldosterone-sensitive transporter in the DCT; also responsive to angiotensin II, insulin, and vasopressin; intracellular trafficking of NCC regulated by WNK kinase phosphorylation; transporter directly inhibited by thiazide diuretics; associated with epithelial sodium (Na+) channel, ENaC |
| SLC26A6 | reabsorption of Cl– in exchange for HCO3– efflux; also involved in oxalate and sulfate anion transport | |
| SCNN1A | ENaC α-subunit | forms a heterotrimeric Na+ channel with the SCNN1B and SCNN1G encoded proteins; the complex localizes with NCC protein in the DCT; mutations in gene result in pseudohypoaldosteronism type 1 |
| SCNN1B | ENaC β-subunit | forms a heterotrimeric Na+ channel with the SCNN1A and SCNN1G encoded proteins; the complex localizes with NCC protein in the DCT; mutations in gene result in pseudohypoaldosteronism type 1 |
| SCNN1G | ENaC γ-subunit | forms a heterotrimeric Na+ channel with the SCNN1A and SCNN1B encoded proteins; the complex localizes with NCC protein in the DCT |
| TRPM6 | transient receptor potential melastatin 6; primarily involved in Mg2+ reabsorption but may transport Ca2+ as well; forms homotetramers and also heterotetramers with TRPM7 | |
| TRPP2 | transient receptor potential polycystin 2; Ca2+ reabsorption transporter | |
| TRPV4 | transient receptor potential vanilloid 4; Ca2+ reabsorption transporter | |
| TRPV5 | transient receptor potential vanilloid 5; expressed in the late DCT region (DCT2); major Ca2+ reabsorption transporter; can form heteromeric complexes with TRPV6; co-localizes with the calcium-binding protein calbindin-D28K | |
| TRPV6 | transient receptor potential vanilloid 6; Ca2+ reabsorption transporter | |
| Apical Membrane Efflux to Tubular Lumen | ||
| SLC9A2 | NHE2: sodium hydrogen (H+) exchanger 2 | efflux of H+ with simultaneous uptake of Na+ |
| SLC26A6 | efflux of HCO3– in exchange for Cl– reabsorption; also involved in oxalate and sulfate anion transport | |
| KCNA1 | Kv1.1 | member of the voltage-gated potassium channel subfamily A; efflux of K+ to the lumen |
Connecting Tubule (CNT) and Collecting Duct
The connecting tubule (CNT) and the collecting duct represent the predominant aldosterone-sensitive region of the distal nephron. The function of aldosterone in this region of the nephron is to ensure that there is appropriate regulation of sodium (Na+) absorption from the glomerular filtrate in order to maintain whole body Na+ balance. The major transporter within the epithelial cells of the CNT and the collecting duct that is responsible for this critical Na+ balance is identified as ENaC (epithelial Na+ channel). The level of expression of the ENaC channel is regulated by aldosterone. The ENaC channel is composed of three subunits (α, β, and γ) that are encoded by three distinct genes that are member of the large family of sodium ion channels. The three genes encoding proteins of the ENaC are SCNN1A (α-subunit), SCNN1B (β-subunit), and SCNN1G (γ-subunit). The transepithelial transport of Na+ within the CNT and collecting duct epithelial cells involves ENaC present in the apical membrane and the renal Na+,K+-ATPase that is distributed in the basolateral membranes of epithelial cells along the nephron. In addition to aldosterone-sensitive Na+ reabsorption, the CNT and the collecting duct are responsible for K+ secretion, contribute to acid (H+) secretion, and also participate in regulation of magnesium and calcium reabsorption.
As described earlier, the epithelial cells of the CNT and collecting ducts (as well as within the distal tubule) are of three distinct types. These cell types are the principal cells and the intercalated cells identified as type A (alpha) and type B (beta). Principal cells reabsorb Na+ and excrete K+. Principal cells are the major cell types that respond to the actions of aldosterone. Type A intercalated cells reabsorb both K+ and HCO3– and they excrete H+. Type B intercalated cells function in opposition to type A cells such that they secrete HCO3– and reabsorb H+. The differences in function between type A and type B intercalated cells is due, in part, to the opposing distributions of various transporter proteins which, for example, places a particular transporter in the apical membrane of a type A cell and the same transporter in the basolateral membrane of a type B cell.
Regulation of potassium balance via cells of the CNT is primarily due to the function of the renal outer medullary potassium (K+) channel identified as ROMK. The ROMK protein is encoded by the KCNJ1 gene. The ROMK channel is present in the apical membranes of principal cells. Water reabsorption is also a critical function of the CNT and collecting duct and is the function of the vasopressin-responsive aquaporin, AQP2. The AQP2 channel is located in the apical membrane of principal cells. Activity of AQP2 is controlled via the actions of the angiotensin 1 receptors (AT1R) following their activation by binding angiotensin II.
Reabsorption and secretion of H+ within the CNT and collecting duct occurs via the actions of the V-ATPase complex (also identified as the H+-ATPase) present in the apical membrane of type A intercalated cells and the basolateral membrane of type B intercalated cells. The V-ATPase is composed of multiple subunits amassed into two distinct domains. The V1 complex is the cytosolic catalytic complex and is composed of eight different subunits encoded by eight distinct genes. The V0 complex is the transmembrane portion of the V-ATPase complex and is composed of at least five subunits encoded by distinct genes.
As indicated above in the discussion of the distal convoluted tubule, active Ca2+ reabsorption is restricted to the late distal convoluted (DCT2) and to the connecting tubules (CNT). Within these regions of the distal nephron approximately 10–15% of the total Ca2+ is reabsorbed with the majority being transported by the apical membrane localized transient receptor potential vanilloid 5 (TRPV5). Following uptake of Ca2+ at the apical membrane, the calcium-binding protein identified as calbindin-D28K, whose synthesis is stimulated by the bioactive form of vitamin D (calcitriol), sequesters the ion and transports it to the basolateral membrane where it can be effluxed back to the blood. This Ca2+ efflux is carried out by either the NCX1 (Na+-Ca2+ exchanger 1: encoded by the SLC8A1 gene) or the PCMA1b (plasma membrane Ca2+ transporter 1b: encoded by the ATP2B1 gene) transporters.
In addition to the actions of vasopressin and aldosterone in the CNT and collecting duct to regulate water, Na+, and K+ balance, this region of the nephron also responds to numerous additional hormonal, chemical, and physical inputs. Some of these inputs include nitric oxide (NO), bradykinin, prostaglandin E2 (PGE2), and ATP.
Table of Connecting Tubule and Collecting Duct Transporters/Channels
| Gene | Common/Other Name | Primary Cell Type | Functions / Comments |
| Basolateral Membrane Uptake from Blood | |||
| SLC4A1 | AE1: anion exchanger 1 | Type A | Cl–/HCO3– exchanger; Cl– absorption from blood in exchange for HCO3– efflux to blood; is an N-terminally truncated isoform of the erythrocyte-specific protein which is also identified as BND3 (erythrocyte band 3 protein) |
| SLC8A1 | NCX1: Na+/Ca2+-exchanger 1 | effluxes Ca2+ to blood in exchange for uptake of Na+ | |
| SLC12A2 | NKCC1: Na+/K+/2 Cl–+ cotransporter type 1 | Type A | uptake of ammonia (NH3) from the blood along with Na+, K+, and Cl– |
| SLC26A7 | Type A | HCO3– in exchange for uptake Cl–; also involved in oxalate and sulfate anion transport | |
| RHBG | Rh blood group antigen B | Type A | the RHBG gene is also identified as the SLC42A2 gene; ammonia (NH3) uptake from blood |
| RHCG | Rh blood group antigen C | Type A | the RHCG gene is also identified as the SLC42A3 gene; ammonia (NH3) uptake from blood; also present in the apical membrane for NH3 secretion |
| Basolateral Membrane Efflux to Blood | |||
| ATP2B1 | PMCA1b: plasma membrane Ca2+ transporter 1b | Type A | ATP-hydrolysis-mediated Ca2+ efflux; the PMCA1b protein isoform is derived from a specific alternatively spliced mRNA from the ATP2B1 gene |
| SLC4A1 | AE1: anion exchanger 1 | Type A | Cl–/HCO3– exchanger; is an N-terminally truncated isoform of the erythrocyte-specific protein which is also identified as BND3 (erythrocyte band 3 protein); Cl– absorption from blood in exchange for HCO3– efflux to blood |
| SLC4A2 | AE2: anion exchanger 2 | Type A | Cl–/HCO3– exchanger; Cl– absorption from blood in exchange for HCO3– efflux to blood |
| SLC4A3 | AE3: anion exchanger 3 | Type A | Cl–/HCO3– exchanger; Cl– absorption from blood in exchange for HCO3– efflux to blood |
| SLC4A9 | AE4: anion exchanger 4 | Type A | Na+ and HCO3– efflux to blood; expressed exclusively in the kidney |
| SLC8A1 | NCX1: Na+/Ca2+-exchanger 1 | effluxes Ca2+ to blood in exchange for uptake of Na+ | |
| SLC12A7 | KCC4: K+/2 Cl– cotransporter 4 | Type B | K+ and Cl– efflux to blood |
| SLC26A7 | Type A | Cl– efflux in exchange for HCO3– uptake; also involved in oxalate and sulfate anion transport | |
| CLCNKB | ClC-Kb: chloride channel Kb | Type A | voltage-gated chloride channel; exclusively expressed in the kidney and inner ear; requires an accessory β-subunit called barttin encoded by the BSND gene; effluxes Cl– ion to the blood; mutations in gene are the cause of Bartter syndrome type 3 |
| KCNJ10 | Kir4.1 | Principal | member of the inwardly-rectifying potassium channel family; coupled to the actions of the Na+,K+-ATPase for efflux of K+ to the blood |
| KCNJ16 | Kir5.1 | Principal | member of the inwardly-rectifying potassium channel family; coupled to the actions of the Na+,K+-ATPase for efflux of K+ to the blood |
| V-ATPase | H+-ATPase | Type B | H+ efflux to blood occurs via V-ATPase present in type B intercalated cells; multisubunit complex composed of a cytoplasmic catalytic (V1) complex of eight proteins and a transmembrane (V0) complex composed of at least five subunits; all subunits of the V0 and V1 complexes are encoded by different genes |
| Apical Membrane Uptake from Tubular Lumen | |||
| ATP4A | H+/K+-ATPase | Principal | α1-subunit of H+/K+-ATPase (often designated HKα1); major transporter involved in K+ reabsorption |
| ATP12A | H+/K+-ATPase | Principal | α2-subunit of H+/K+-ATPase (often designated HKα2); major transporter involved in K+ reabsorption |
| ATP4b | H+/K+-ATPase | Principal | β-subunit of H+/K+-ATPase; major transporter involved in K+ reabsorption |
| AQP2 | aquaporin 2 | Principal | major vasopressin-responsive water channel in the CNT and collecting duct |
| SLC26A6 | Type A | reabsorption of Cl– in exchange for HCO3– efflux; also involved in oxalate and sulfate anion transport | |
| SCNN1A | ENaC: epithelial sodium channel; α-subunit | Principal | forms a heterotrimeric non-voltage gated ameloride-sensitive Na+ channel with the SCNN1B and SCNN1G encoded proteins; forms the primary Na+ transporter in the connecting tubule and collecting ducts; loss-of-function mutations in gene result in pseudohypoaldosteronism type 1 (neonatal salt-wasting hyperkalemia); gain-of-function mutations in gene result in Liddle syndrome (characterized by hypertension and hypokalemia) |
| SCNN1B | ENaC: epithelial sodium channel; β-subunit | Principal | forms a heterotrimeric non-voltage gated ameloride-sensitive Na+ channel with the SCNN1A and SCNN1G encoded proteins; forms the primary Na+ transporter in the connecting tubule and collecting ducts; loss-of-function mutations in gene result in pseudohypoaldosteronism type 1 (neonatal salt-wasting hyperkalemia); gain-of-function mutations in gene result in Liddle syndrome (characterized by hypertension and hypokalemia) |
| SCNN1G | ENaC: epithelial sodium channel; γ-subunit | Principal | forms a heterotrimeric non-voltage gated ameloride-sensitive Na+ channel with the SCNN1A and SCNN1B encoded proteins; forms the primary Na+ transporter in the connecting tubule and collecting ducts |
| SLC4A8 | NDCBE: Na+-driven Cl–/HCO3– exchanger | Type B | Na+ and HCO3– reabsorption coupled to Cl– efflux; protein is also identified as NBC3 |
| SLC26A4 | Pendrin | Type B | Cl– uptake coupled to HCO3– efflux; originally identified as mutated gene in Pendred syndrome which is characterized by hypothyroidism, goiter, and sensorineural deafness |
| TRPC3 | transient receptor potential canonical 3; collecting duct only; Ca2+ reabsorption transporter | ||
| TRPC6 | transient receptor potential canonical 6; collecting duct only; Ca2+ reabsorption transporter | ||
| TRPV4 | transient receptor potential vanilloid 4; Ca2+ reabsorption transporter | ||
| TRPV5 | transient receptor potential vanilloid 5; expressed in late DCT (DCT2); Ca2+ reabsorption transporter; co-localizes with the calcium-binding protein calbindin-D28K | ||
| TRPV6 | transient receptor potential vanilloid 6; expressed in late DCT (DCT2); Ca2+ reabsorption transporter | ||
| Apical Membrane Efflux to Tubular Lumen | |||
| SLC4A8 | NDCBE: Na+-driven Cl–/HCO3– exchanger | Type B | Cl– efflux coupled to Na+ and HCO3– reabsorption; protein is also identified as NBC3 |
| SLC26A4 | Pendrin | Type B | Cl– uptake coupled to HCO3– efflux |
| SLC26A6 | Type A | efflux of HCO3– in exchange for Cl– reabsorption; also involved in oxalate and sulfate anion transport | |
| SLC26A9 | Principal | efflux of Cl– | |
| KCNJ1 | ROMK: renal outer medullary potassium channel | Principal | K+ efflux to lumen; members of the KCNJ family of inwardly rectifying voltage-gated potassium channels are also identified a Kir channels with ROMK being Kir1.1 (three isoforms due to alternative splicing: Kir1.1a, Kir1.1b, and Kir1.1c); aldosterone activates ROMK by inducing its phosphorylation by SGK1 (serum/glucocorticoid regulated kinase 1) which results in potassium secretion; mutations in gene are the cause of Bartter syndrome type 2 |
| RHCG | Rh blood group antigen C | Type A | the RHCG gene is also identified as the SLC42A3 gene; ammonia (NH3) efflux to urine; also present in the basolateral membrane for uptake of NH3 from the blood |
| V-ATPase | H+-ATPase | Type A | H+ efflux to urine occurs via V-ATPase present in type A intercalated cells; multisubunit complex composed of a cytoplasmic catalytic (V1) complex of eight proteins and a transmembrane (V0) complex composed of at least five subunits; all subunits of the V0 and V1 complexes are encoded by different genes |
WNK Kinases and Regulation of Renal Transport
The WNK kinases are so-called because the canonical lysine residue, normally present in subdomain II of the ATP binding site of the catalytic site of all other Ser/Thr kinases, is found in subdomain I. Hence the derivation of With No K (K is single letter code for lysine). WNK kinases are expressed in numerous tissues where they exert effects on solute transport, cell growth, and nerve transmission. WNK kinases function as members of large kinase networks that include the kinases SGK1 (serum and glucocorticoid inducible kinase 1) and STK39 (Ser/Thr kinase 39; also identified as SPAK for Ste20-related Proline Alanine-rich Kinase).
Humans express four WNK genes identified as WNK1, WNK2, WNK3, and WNK4. The WNK1 gene is located on chromosome 12p13.33 and is composed of 31 exons that generate four alternatively spliced mRNAs with the 2382 amino acid isoform 1 being the most common. Mutations in the WNK1 gene are associated with pseudohypoaldosteronism type IIA (also known as Gordon syndrome) and hereditary sensory neuropathy type II. The WNK2 gene is located on chromosome 9q22.31 and is composed of 38 exons that generate two alternatively spliced mRNAs both of which encode distinct protein isoforms. The WNK3 gene is located on the X chromosome (Xp11.22) and is composed of 25 exons that generate two alternatively spliced mRNAs both of which encode distinct protein isoforms. The WNK4 gene is located on chromosome 17q21.2 and is composed of 18 exons that generate two alternatively spliced mRNAs both of which encode distinct protein isoforms. Mutations in the WNK4 gene are associated with pseudohypoaldosteronism type IIB.
Of significance to renal function is the fact that the WNK kinases, particularly WNK1 and WNK4, regulate the activity of several ion transporters. The WNK kinases are responsible for modifying transporter activity in response to varying physiological demands. The activity of the WNK kinases leads to the regulation of sodium and potassium concentrations in the blood and urine and thus, affects blood pressure. The transporters regulated by WNK kinases include the sodium potassium chloride cotransporter type 2 (NKCC2: encoded by the SLC12A1 gene), the sodium chloride cotransporter (NCC: encoded by the SLC12A3 gene), the epithelial sodium channel (ENaC: composed of three subunits encoded by the SCNN1A, SCNN1B, and SCNN1G genes), and the aldosterone-responsive renal outer medullary potassium channel (ROMK: encoded by the KCNJ1 gene).
The activity of WNK kinases is affected by both hypovolemia and hyperkalemia. Under conditions leading to hypovolemia the extracellular fluid volume needs to be tightly regulated in order to ensure proper regulation of blood pressure and resultant organ perfusion. Within the kidney hypovolemia activates the renin angiotensin system and enhances aldosterone secretion from renal cortex zona glomerulosa cells. Renin, released from the juxtaglomerular cells, cleaves angiotensinogen to angiotensin I which is then cleaved to the bioactive form, angiotensin II, via the action of angiotensin converting enzyme (ACE).
Within the kidney tubules angiotensin II stimulates Na+ and Cl– reabsorption, K+ secretion, and water retention. Aldosterone exerts its effect predominantly within the late distal convoluted tubule (DCT2), the connecting tubule, and the collecting duct. Within these regions of the nephron aldosterone induces the expression of the Na+/K+-ATPase subunit genes (ATP1A1 and ATP1B1), the genes encoding the subunits (SCNN1A, SCNN1B, and SCNN1G) of the epithelial sodium channel (ENaC), and the SLC12A3 gene (encoding the NCC transporter). During hyperkalemia potassium secretion must be enhanced in order to prevent cardiac conductance and neuromuscular complications. The enhanced K+ secretion is mediated by the effects of aldosterone.
Given that aldosterone can exert different effects on sodium and potassium transport in the kidney tubule under differing physiological conditions these differing effects have been termed the aldosterone paradox. These differences in response, at the level of aldosterone, to hypovolemia and hyperkalemia are due to the fact that only under conditions of hypovolemia is there an accompanying activation of the renin angiotensin system. Angiotensin II reverses the inhibitory effects exerted by WNK4 on NCC trafficking to the membrane. This allows NCC to be transported to the membrane where it can participate in Na+ and Cl– transport leading to increased Na+ reabsorption in the DCT. The increased Na+ uptake reduces the amount of the ion reaching the collecting duct, thereby limiting Na+-coupled K+ efflux from the collecting duct.
WNK4 normally phosphorylates and stimulates the activity of ROMK. Angiotensin II inhibits ROMK by activating phosphorylation and inhibition of WNK4 as well as activating phosphorylation (by the tyrosine kinase SRC) and inhibition of ROMK itself. The effects of angiotensin II, therefore, collectively lead to Na+ reabsorption while preventing K+ efflux. Conversely, during conditions of hyperkalemia there is no angiotensin II effect and the effects of aldosterone alone result in the stimulation of K+ secretion directly. The activity of both WNK1 and WNK4 are increased in the presence of high dietary potassium which results in the inhibition of NCC and the activation of ROMK. These combined effects lead to enhanced Na+ reabsorption in exchange for K+ efflux.
Renal Transporter Pharmacology
Numerous drugs, used for a variety of conditions, in particular cardiovascular disturbances, have been developed whose effects are exerted, in part or in whole, through modification of renal tubular functions. The largest class of renal acting drug is the diuretics. Diuretic drugs function in a number of different capacities and at differing locations along the nephron but all result in diuresis (increased production of urine) involving increased water excretion.
The diuretic drugs are used in the treatment of conditions such as hypertension, heart failure, glaucoma, and liver cirrhosis. In addition, certain diuretics, such as the carbonic anhydrase inhibitors (e.g. acetazolamide), can be used in the treatment renal lithiasis (kidney stones) through their ability to alkalize the urine.
Although used in the treatment of hypertension, some diuretic drugs exert effects on blood pressure at doses that are independent of their diuretic effects (e.g. loop diuretics and thiazide diuretics). The diuretic drugs include the loop diuretics, the thiazide diuretics, the potassium-sparing diuretics, the carbonic anhydrase inhibitor diuretics, and the osmotic diuretics.
Another important class of renal acting drugs is the uricosuric class. Drugs that are classified as uricosuric result in increased uric acid excretion via the kidneys and are, therefore, useful in the treatment of hyperuricemia and gout. Not all drugs in a given class are described in the following paragraphs, only representative examples and their modes of action.
Loop Diuretics
The loop diuretic class of drug includes furosemide, bumetanide, torsemide, and ethacrynic acid (also called etacrynic acid). The range of uses for loop diuretics is broad and includes generalized edema, pulmonary edema, hypertension, heart failure, hypercalcemia, oliguria (diminished urine output), ascites, and anion overload. These drugs act by decreasing Na+ reabsorption principally in the thick ascending limb (TAL) of the loop of Henle.
The primary site of action of loop diuretics is the Na+/K+/2 Cl– cotransporter 2 (NKCC2: encoded by the SLC12A1 gene) which is present in the apical membrane of the epithelial cells in this region of the nephron. In addition to blocking Na+ reabsorption, the loop diuretics also lead to loss K+ reabsorption due to blockade of NKCC2 activity. The reduced K+ reabsorption can result in muscle cramping, weakness, and pain.
Torsemide is used primarily in the management of the edema associated with congestive heart failure. The utility of this drug is that its’ diuretic effect is greater than furosemide at doses of similar potency and the accompanying K+ loss is reduced with torsemide compared to furosemide.
Ethacrynic acid is not a sulfonamide derivative and thus is useful in patients with allergies to sulfa drugs. However, high dose treatments with ethacrynic acid can result in ototoxicity (permanent hearing loss).
Thiazide Diuretics
Thiazide diuretics are derived from the parent compound, benzothiadiazine. Another group of drugs that are not structurally related to the thiazides but exert effects in a similar manner are referred to as the thiazide-like diuretics. The thiazides include altizide, chlorothiazide, cyclothiazide, hydrochlorothiazide, and hydroflumethiazide. The thiazide-like drugs include indapamide, metolazone, clopamide, and chlorthalidone.
The range of uses for the thiazide and thiazide-like diuretics includes hypertension, congestive heart failure, edema, nephrogenic diabetes insipidus, and nephrocalcinosis (renal stones composed of calcium oxalate or calcium phosphate). The thiazides function to inhibit Na+ and Cl– reabsorption in the distal convoluted tubule, DCT. These drugs exert their effects by inhibiting the thiazide-sensitive Na+/Cl– cotransporter identified as NCC (also identified as NCCT: encoded by the SLC12A3 gene).
The thiazide diuretics also lead to increased Ca2+ reabsorption in the distal convoluted tubule which in turn makes them useful drugs for the prevention of calcium oxalate stones. This Ca2+ effect of the thiazides is an indirect consequence of their inhibition of the apical membrane-associated NCC transporter. The reduced reabsorption of Na+ from the lumen of the tubule activates the basolateral membrane-associated Na+/Ca2+ exchanger identified as NCX1 (encoded by the SLC8A1 gene). The function of NCX1 is to efflux Ca2+ back to the blood in exchange for the uptake of Na+ into the renal epithelial cell. Due to the thiazide-induced inhibition of NCC at the apical membrane, the uptake of Na+ from the blood is increased concomitant with increased Ca2+ efflux to the blood. As the intracellular Ca2+ concentration drops the activity of the major apical membrane Ca2+ transporter, TRPV5, is enhanced, thus resulting in reduced luminal Ca2+ and a reduced likelihood for renal lithiasis.
The reduction in glomerular filtrate Ca2+ concentration and the increased reabsorption of Ca2+ effects of the thiazides allows them to be used in the treatment of hypocalcemia and in the prevention of calcium oxalate stones associated with Dent disease.
Potassium-Sparing Diuretics
The potassium-sparing diuretics are so-called because they exert their diuretic effects without altering K+ reabsorption, therefore there is no accompanying reduction in circulating K+ as is the case with the loop diuretics. The modes of action of the potassium-sparing diuretics allow them to be divided into two classes, the epithelial sodium channel blockers (e.g. amiloride and triamterene) and the aldosterone antagonists. The latter class can be further divided into the drugs that interfere with the effects of aldosterone by blocking its interaction with its intracellular receptor (e.g. spironolactone) and those that block the production of aldosterone by interfering with the renin-angiotensin-aldosterone system (RAAS).
Amiloride and triamterene exert their effects by blocking the amiloride-sensitive sodium channel present in the apical membrane of epithelial cells in the late distal convoluted tubule (DCT2) and the connecting tubule (CNT). This sodium channel is identified as ENaC (epithelial Na+ channel) the represents the major apical membrane Na+ channel present in principal cells of the CNT and collecting duct. The ENaC transporter functions as a heterotrimeric complex composed of three subunits (α, β, and γ) encoded by three distinct genes (SCNN1A, SCNN1B, and SCNN1G). Within the CNT and collecting duct Na+ reabsorption results in an increase in the negative charge within the tubular lumen. The electrochemical gradient normally drives K++ efflux from the epithelial cell into the lumen. The inhibition of Na+ reabsorption thus, prevents the normally associated K+ efflux to the lumen, hence the potassium-sparing effects of these drugs.
Spironolactone exerts its effects by acting as an antagonist of the aldosterone receptor, thereby blocking the transcriptional regulation normally exerted by aldosterone. Aldosterone is produced and released from adrenal cortical cells of the zona glomerulosa in response to decreased plasma Na+ concentration. Aldosterone exerts its effect predominantly within the late distal convoluted tubule (DCT2), the connecting tubule, and the collecting duct. Within these regions of the nephron aldosterone induces the expression of the Na+/K+-ATPase subunit genes (ATP1A1 and ATP1B1), the genes encoding the subunits (SCNN1A, SCNN1B, and SCNN1G) of the epithelial sodium channel (ENaC), and the SLC12A3 gene (encoding the NCC transporter). The net effect of the induction of these transporter genes by aldosterone is enhanced Na+ reabsorption as a function of the apical membrane localized ENaC and NCC transporters and delivery to the blood via the action of the basolateral Na+/K+-ATPase. Secondary to the Na+ uptake is efflux of K+ to the tubular lumen.
The aldosterone induced Na+ reabsorption indirectly influences water retention as water movement follows Na+ movement across the membrane as a means to maintain osmolarity. However, because only small amounts of Na+ are reabsorbed in the distal nephron spironolactone functions as a weak diuretic. It is often combined with other diuretics to enhance efficiency. Since aldosterone action leads to K+ secretion blocking its function with spironolactone results in potassium-sparing diuresis.
Carbonic Anhydrase Inhibitor Diuretics
Carbonic anhydrases (CA) are a large family of zinc metalloenzymes that catalyze the reversible formation and dehydration of carbonic acid (H2CO3). In the formation direction carbonic anhydrases catalyze the condensation of CO2 and H2O forming carbonic acid and in the opposite direction release H+ and HCO3– (bicarbonate).
Humans express 15 CA isoforms with 12 of these exhibiting functional enzymatic activity. Within the kidney the major site of action of the carbonic anhydrase inhibitors is within the proximal tubule. The CA II isoform is the predominant CA isoform found in the apical membrane of proximal tubule epithelial cells.
The CA inhibitors are members of the large sulfonamide class of compounds with the two major drugs in this class being acetazolamide and methazolamide. The major uses for CA inhibitors include the treatment of glaucoma, the treatment of metabolic alkalosis due to hypokalemia or high aldosterone levels, alkalizing urine to enhance uric acid and cystine secretion in order to prevent renal calculi, and in the treatment of acute mountain (high altitude) sickness. The CA inhibitors promote alkaline diuresis and hyperchloremic metabolic acidosis and as such the latter effect is one of the adverse consequences of the use of CA inhibitors.
Osmotic Diuretics
Osmotic diuretics inhibit the reabsorption of water and Na+ resulting in extracellular fluid expansion. The principal members of this drug class include mannitol and isosorbide. Within the kidneys the osmotic diuretics act primarily at the level of the proximal tubule and the descending limb of the loop of Henle. Because the osmotic diuretics alter the osmolarity of the renal fluid they exert a secondary effect at opposing the actions of vasopressin in the connecting tubule (CNT) and collecting duct. Osmotic diuretics also exert effects in other parts of the body and for this reason they can be, and are, used in the treatment of conditions such as intracranial pressure as in hydrocephalus and intraocular pressure as in glaucoma.
Calcium-Sparing Diuretics
Calcium-sparing diuretics is a term that does not apply to a distinct class of diuretic drug but is often used when comparing the actions of the loop diuretics and the thiazide diuretics. The loop diuretics result in significant Ca2+ secretion which can lead to an increased risk for reduction in bone density. In contrast, the thiazide diuretics result in a decrease in urinary loss of Ca2+ and are, therefore, often referred to as calcium-sparing diuretics. The potassium-sparing diuretics result in some Ca2+ loss but not to the extent of the loop diuretics so they too are often referred to as calcium-sparing diuretics.
Uricosuric Drugs
Hyperuricemia is defined as a serum urate concentration exceeding 7.0mg/dL in men and 6.0mg/dL in women. However, it should be noted that serum urate concentrations vary markedly among different populations and the values are influenced by such things as age, sex, ethnicity, body weight and the surface area of the body. Hyperuricemia can result from either excess uric acid production or reduced secretion or a combination of both mechanisms. Gout is the pathology resulting from excess uric acid production and/or reduced uric acid secretion. Gout does not occur in the absence of hyperuricemia. Primary gout is a biochemically and genetically heterogeneous disorder resulting from inborn metabolic errors that alter uric acid homeostasis. Gouty arthritis is the most painful manifestation of gout and is caused when urate crystals interact with neutrophils triggering an inflammatory response.
There are two main pharmacological approaches to the treatment of hyperuricemia, one is to reduce uric acid production and the other is to reduce renal reabsorption of uric acid. Any drug that increases the renal secretion of uric acid is classified as a uricosuric. Several uricosuric drugs exert their effects by interferring with uric acid transport primarily via proximal tubule reabsorption.
There are several uric acid transporters, however, the major urate transporter is URAT1 (encoded by the SLC22A12 gene) located in the apical membrane of proximal tubule epithelial cells. Lesinurad is a uricosuric agent that functions by directly inhibiting proximal tubule URAT1 as well as another proximal tubule uric acid reabsorption transporter, OAT4 (encoded by the SLC22A11 gene). However, the sale and use of lesinurad was discontinued in the US in 2019.
Another important uricosuric drug is probenecid which also acts to inhibit proximal tubule uric acid reabsorption. Because probenecid affects the activities of several proximal tubule transporters it can also be used in conjunction with certain other drugs to inhibit their secretion and thus, increase their effective serum concentrations.
Transport of urate via SLC22A12 is selectively inhibited by lactate, succinate, β-hydroxybutyrate, and acetoacetate. Urate reabsorption is coupled to renal lactate reabsorption at the apical membrane of the proximal tubule via interactions between SLC22A12 and the lactate transporters encoded by the SLC5A8 and SCL5A12 genes. Due to this interaction lactic acidemia impairs urate reabsorption and excretion while hyperuricemia results in impaired lactate excretion and reabsorption. As a result of these transporter interactions the use of probenecid in the treatment of hyperuricemia can lead to exacerbation of pre-existing lactic acidemia.
Two additional uricosuric drugs, sulfinpyrazone and benzbromarone are no longer used in the US due to toxicities.
Renal Transporters and Disease
Renal Tubular Acidosis, RTA
Metabolic acidosis can result from the accumulation of acids, predominantly H+, or from the excess loss of bicarbonate (HCO3–). Normal determination of metabolic acidosis is made from assessment of the plasma anion gap determined from the following: [Na+ – (Cl– + HCO3–)].
Renal tubular acidosis (RTA) represents a form of hyperchloremic metabolic acidosis and refers to pathologies that arise as a result of the failure of the kidneys to properly transport acid (H+) into the tubular lumen for its excretion in the urine, or to retain HCO3– under conditions of normal or slightly impaired glomerular filtration rate (GFR). These conditions result in the accumulation of acid within the body resulting in the associated metabolic acidosis.
There are three commonly defined types of RTA identified as type 1, type 2, and type 4. A type 3 RTA has been defined but it is no longer used as a common designation as it is a rare and transiently presenting form of RTA that shares features of both type 1 and type 2 RTA. In addition, what is referred to as type 4 RTA is not truly a disorder of tubular transport processes but the metabolic acidosis results secondarily to an underlying hypoaldosteronism. Secondary RTA can also result in humans due to the underlying pathologies of several disorders such as systemic lupus erythematosus (SLE), sickle cell disease, and Sjögren syndrome, or due to certain drug therapies or the ingestion of toxins.
Type 1 Renal Tubular Acidosis
Type 1 RTA is also referred to as distal RTA given that the location of the defect in H+ transport (efflux) is in the medullary collecting ducts. Type 2 RTA is also referred to as proximal RTA since the defect in acid regulation resides within the proximal tubule. Type 1 RTA represents the classic form of this disorder as it was the first one characterized. The cells that are at the root cause of type 1 RTA are the type A (alpha) intercalated cells present in the medullary collecting ducts. The failure to excrete H+ results in an associated failure to reabsorb K+ resulting in combined metabolic acidosis and hypokalemia.
The symptoms of type 1 RTA include growth impairment, dehydration, hemolytic anemia, nephrocalcinosis, urolithiasis, and deafness. The occurrence of hemolytic anemia and/or deafness in RTA type 1 is dependent on the gene that is affected resulting in the disorder. Three different genes have been shown to be mutated in patients with type 1 RTA. These gene include SLC4A1, ATP6V1B1, and ATP6V0A4. The SLC4A1 gene encodes the anion exchanger 1 (AE1) protein. The ATP6V1B1 gene encodes the B1 subunit of the V1 complex of V-ATPase (also identified as H+-ATPase). The ATP6V0A4 gene encodes the A4 subunit of the V0 complex of V-ATPase. The SLC4A1 gene is located on chromosome 17q21.31 and is composed of 21 exons that encode a 911 amino acid protein. The ATP6V1B1 gene is located on chromosome 2p13.3 and is composed of 15 exons that encode a 513 amino acid protein. The ATP6V0A4 gene is located on chromosome 7q34 and is composed of 24 exons that generate three alternatively spliced mRNAs each of which encode the same 840 amino acid protein.
Mutations in the SLC4A1 gene that are associated with type 1 RTA are inherited in an autosomal dominant or recessive manner while mutations in both the ATP6V1B1 and ATP6V0A4 genes are inherited as autosomal recessive mutations. Mutations in the ATP6V1B1 gene result in type 1 RTA associated with sensorineural deafness, whereas mutations in ATP6V0A4 gene are not associated with deafness.
Type 1 RTA is the most common form of RTA. A correct diagnosis of type 1 RTA is made through an accurate determination of blood pH and HCO3– concentration with the use of a blood gas analyzer. The measurement of pH in fresh urine samples must be carried out with a pH meter in order to get an accurate value. In addition, the assessment of titratable acid in the urine, especially ammonium ion (NH4+), must be determined during acidosis.
Type 2 Renal Tubular Acidosis
Type 2 RTA results from the inability of proximal tubular cells to reabsorb HCO3– resulting in urinary excess (wasting) and consequent metabolic acidosis. Since the function of the connecting tubule and collecting duct intercalated cells is not impaired in type 2 RTA the level of acidosis is less severe. The occurrence of type 2 RTA is rare and is associated with mutations in several different genes.
Type 2 RTA is most often associated with a generalized proximal tubular dysfunction known as Fanconi syndrome which is a disorder in which all reabsorption processes in the proximal tubule are impaired. In addition to the generalized proximal tubule reabsorption impairment, type 2 RTA is often associated with neurological and ocular anomalies, dependent upon the gene defect.
To date mutations in at least 12 different genes have been shown to contribute to type 2 RTA. These genes encode various different types of proteins including transporters and enzymes such as the voltage-gated chloride channels encoded by the CLCN5 and CLCN7 genes, the Na+-bicarbonate cotransporter 1 (NBC1: encoded by the SLC4A4 gene), the glucose transporter GLUT2 (encoded by the SLC2A2 gene), the galactose-1-phosphate uridylyltransferase enzyme (encoded by the GALT gene), and the copper transporter beta polypeptide (encoded by the ATP7B gene). Mutations in the GALT gene are the causes of classic galactosemia and mutations in the ATP7B gene are the cause of Wilson disease.
Type 3 Renal Tubular Acidosis
As indicated the use of the term type 3 RTA is no longer a standard in the comparisons of various forms of RTA. However, what was originally termed type 3 RTA represents a disorder that shares symptoms of both type 1 (distal) and type 2 (proximal) RTA. The principal symptoms of type 3 RTA include osteopetrosis (unusually dense bones, also known as stone bone disease) and cerebral calcification. Type 3 RTA most commonly results from mutations in the carbonic anhydrase 2 (CA2) gene. The CA2 encoded enzyme is most often designated CAII or CA-II. The CAII enzyme is a cytoplasmic protein that, within the renal epithelial cell, forms a complex with several membrane bicarbonate transporters including AE1 (encoded by the SLC4A1 gene), NBC1 (Na+-bicarbonate cotransporter 1: encoded by the SLC4A4 gene), NBC3 (Na+-bicarbonate cotransporter 3: encoded by the SLC4A8 gene), and the SLC26A6 encoded protein, as well as with the proton antiporter, NHE1 (Na+-H+ exchanger 1: encoded by the SLC9A1 gene).
Type 4 Renal Tubular Acidosis
Although there is a designation defined as type 4 RTA, this disorder is not a classical form of RTA in the sense that the clinical syndrome is unlike those of the more classical RTA, type 1 and type 2. Type 4 is associated with mild metabolic acidosis that results from a reduction in proximal tubular ammonium ion efflux (secretion) secondary to hypoaldosteronism. The hypoaldosteronism can be the result of decreased aldosterone production and secretion from the zona glomerulosa cells of the renal cortex or due to impaired responses to aldosterone. Since the cardinal feature of type 4 RTA is hyperkalemia, in the presence of normal urine acidification, the disorder is more appropriately identified as renal tubular hyperkalemia.
Bartter Syndrome
Bartter syndrome represents a disease that belongs to a family of related disorders of renal tubular function that was first described in 1962 by the American endocrinologist, Frederick Crosby Bartter. Specifically Bartter syndrome is a rare inherited form of salt-losing hypokalemic tubulopathy (SLT). Bartter syndrome, like the related Gitelman syndrome described below, is characterized by defective tubular epithelial cell electrolyte transport. In fact there are five identified forms of Bartter syndrome defined in part by clinical characteristics and by the gene whose function is defective in each type. These tubular defects reside in the thick ascending limb (TAL) of the loop of Henle (defined as loop disorders), the distal convoluted tubule, DCT (defined as DCT disorders), or the defects are in both regions and are thus, termed combined disorders. The characteristic feature of hypokalemic alkalosis in Bartter and Gitelman syndromes distinguishes these TAL and DCT disorders from other salt-losing tubulopathies resulting from electrolyte transport defects in other segments of the nephron. The differences are due, in part, from the enhanced compensatory Na+ reabsorption, coupled to K+ efflux, that occurs in the aldosterone-sensitive distal portions of the nephron. The overall incidence of all forms of Bartter syndrome is estimated to be 1 in 100,000.
Type 1 Bartter Syndrome
Bartter syndrome type 1 is a prenatal manifesting form of the disorder. This form of the syndrome is a loop disorder (also referred to as L1 type) as the defective gene affects functions predominantly in the TAL. The gene responsible for type 1 Bartter encodes the NKCC2 protein (Na+/K+/2 Cl– cotransporter 2). The gene encoding this transporter is identified as SLC12A1 which is located on chromosome 15q21.1 and is composed of 29 exons that generate two alternatively spliced mRNAs. The SLC12A1 mRNAs both encode proteins of the same length 1099 (amino acids) but that contain different amino acids at positions 214-238.
The characteristic features of type 1 Bartter syndrome are severe natriuresis, hypercalciuria and magnesuria. The inability to concentrate the urine results in hyponatremia, hypochloremia, hypokalemia, metabolic alkalosis, and low blood pressure. Bartter syndrome type I is also characterized by plasma renin levels that are 10-30 times higher than the upper limit for a patients age with aldosterone levels approximately 3 times above the upper limit for age. Elevated plasma renin levels are the more significant hormonal differences in these patients compared with unaffected individuals. The hypokalemia in type 1 Bartter syndrome results from the elevated aldosterone which increases the expression of the genes that encode the epithelial Na+ channel (ENaC) in the DCT. The resultant increased level of ENaC in the membrane results in increased Na+ reabsorption with concomitant increased K+ efflux via the ROMK channel.
If polyhydramnios (excessive amniotic fluid in the amniotic sac) is present between 24 and 38 weeks gestation type 1 Bartter syndrome is strongly suspected. Bartter syndrome type 1 is inherited as an autosomal recessive disease. The incidence of SLC12A1 heterozygous mutations is 1/360.
Type 2 Bartter Syndrome
Bartter syndrome type 2 is a neonatal manifesting form of the disorder. This form of the syndrome is a loop disorder (also referred to as L2 type) as the defective gene affects functions predominantly in the TAL. The gene responsible for type 2 Bartter encodes the renal outer medullary K+ transporter identified as ROMK. ROMK is encoded by the KCNJ1 gene. The KCNJ1 gene is located on chromosome 11q24.3 and is composed of 5 exons that generate five alternatively spliced mRNAs encoding a total of three different protein isoforms. The ROMK protein is a member of the inwardly rectifying K+ channel proteins that are also designated Kir with ROMK being Kir1.1 (three isoforms of ROMK result from alternative splicing: Kir1.1a, Kir1.1b, and Kir1.1c).
The characteristic features of type 2 Bartter syndrome are similar to those of type 1 Bartter except that there is a transient hyperkalemia in the early post-natal period. This transient hyperkalemia is due to the defective function of ROMK. In these patients the hyperkalemia is eventually resolved via the action of calcium-activated potassium channels (commonly referred to as big K+, BK channels or MaxiK channels) within the DCT. Mutations in the KCNJ1 gene are the most common among all individuals with Bartter syndrome. All characterized KCNJ1 mutations reside within exon 5. Bartter syndrome type 2 is inherited as an autosomal recessive disease.
Type 3 Bartter Syndrome
Bartter syndrome type 3 is called classic Bartter syndrome (also called postnatal Bartter syndrome). This form of the syndrome is a DCT disorder (also referred to as DC2 type) as the defective gene affects functions predominantly in the DCT. The gene responsible for classic Bartter syndrome encodes a member of the voltage-gated Cl– channels identified as ClC-Kb. The ClC-Kb protein is encoded by the CLCNKB gene. The CLCNKB gene is located on chromosome 1p36.13 and is composed of 20 exons that generate two alternatively spliced mRNAs each encoding a distinct protein isoform. The CLCNKB encoded Cl– channel functions with an accessory β-subunit protein called barttin (encoded by the BSND gene). Mutations in the gene encoding the barttin protein result in type 4 Bartter syndrome.
There is a great deal of phenotypic variability in patients with type 3 Bartter syndrome ranging from potentially lethal dehydration and hypotension during the first year of life to polyuria, hypocalciuria and hypomagnesemia later in life that resembles the symptoms of Gitelman syndrome. Bartter syndrome type 3 is inherited as an autosomal recessive disease.
Type 4 Bartter Syndrome
Bartter type 4 is also known as antenatal Bartter syndrome with sensorineural deafness. This form of the syndrome is classified as a combined form of the disorder and designated L-DC2. The gene responsible for type 4 Bartter syndrome encodes the common accessory β-subunit of the voltage-gated Cl– channels identified as ClC-Ka and ClC-Kb (the CLCNK channels). This β-subunit is encoded by the BSND gene and the encoded protein is called barttin. The BSND gene is located on chromosome 1p32.3 and is composed of 4 exons that encode a 320 amino acid protein.
Type 4 Bartter syndrome is the most severe form of disease and is characterized by excessive polyhydramnios, premature delivery, and early failure to thrive. Most type 4 Bartter patients manifest renal failure due to renal tissue damage and infiltration of mononuclear cells. The severity of type 4 Bartter is due to the fact that the barttin protein is required for the function of both the ClC-Ka and ClC-Kb chloride transporters which function in the thin and thick ascending limbs of the loop of Henle and the DCT. These Cl– transport defects leading to loss the concentrating capacity of the nephron as well as to reduced Na+ reabsorption in the more distal regions of the nephron. The sensorineural deafness in type 4 Bartter syndrome is due to the loss of ClC-Ka and ClC-Kb function in the inner ear. Atypical type 4 Bartter syndrome has been shown to be the result of the digenic inheritance of mutations in both the CLCNKA (encoding ClC-Ka) and CLCNKB (encoding ClC-Kb) genes.
Type 5 Bartter Syndrome
Type 5 Bartter syndrome is also known as hypocalcemia with Bartter-like syndrome. This form of the syndrome is a DCT disorder (also referred to as DC4 type) as the defective gene affects functions predominantly in the DCT. The gene responsible for type 5 Bartter syndrome encodes the calcium sensing receptor (CaSR) and identified as CASR. The CASR gene is located on chromosome 3q13.33–q21.1 and is composed of 11 exons that generate two alternatively spliced mRNAs that encode two distinct protein isoforms. The mutations in the CASR gene that are associated with type 5 Bartter are all activating mutations and they are inherited in an autosomal dominant manner. The CASR gene is expressed only in the parathyroid gland and the kidney.
Within the kidney the gene is expressed in several regions of the nephron with some cells presenting the protein in the apical membrane and others in the basolateral membrane. Within the TAL, the CaSR protein is present in the basolateral membrane. The normal function of this receptor is to respond to increased Ca2+ in the blood and, upon receptor activation, lead to inhibition of Na+, Cl–, Ca2+ and Mg2+ (as well as other divalent cations) reabsorption from the tubular lumen. The signal transduction pathways activated by CaSR lead to inactivation of the transporters NKCC2 (encoded by the SLC12A1 gene), ROMK (encoded by the KCNJ1 gene) and Na+/K+-ATPase resulting in decreased reabsorption of Ca2+ and Mg2+. The activating mutations in the CASR gene, therefore, result in excessive inhibition of all three transporters. Activating mutations in CaSR also affect K+ balance via inhibition of the inwardly-rectifying potassium channels identified as Kir4.1 and Kir5.1 encoded by the KCNJ10 and KCNJ16 genes, respectively. These K+ transporters are present in the basolateral membrane of the TAL, the DCT, and the connecting tubule cells where they efflux K+ out of the cell into the blood.
The normal function of the KCNJ10 and KCNJ16 encoded transporters is required to establish the electrochemical gradient necessary for efflux of Cl–. Therefore, activating mutations in the CaSR lead to excessive inhibition of K+ efflux resulting in intracellular Cl– accumulation. Elevated Cl– is what leads to inactivation of the NKCC2 transporter via a WNK-mediated pathway.
Patients with type 5 Bartter syndrome initially present with hypocalcemia with tetany and low levels of parathyroid hormone, PTH. Later in life patients experience hypokalemia, hypomagnesemia, metabolic alkalosis as well as elevated circulating levels of renin and aldosterone.
Gitelman Syndrome
Gitelman syndrome, which is also referred to as familial hypokalemic hypomagnesemia, represents one of the most common inherited renal tubulopathies. Gitelman syndrome, like Bartter syndrome characterized by hypokalemic metabolic acidosis but is also associated with significant hypomagnesemia and hypocalciuria. Gitelman syndrome is classified as a DCT disorder (also referred to as DC1 type) as the defective gene affects functions predominantly in the DCT. Mutations in the SLC12A3 gene which encodes the Na+/Cl– cotransporter identified as NCC (also identified as NCCT) are the cause of Gitelman syndrome. The SLC12A3 gene is located on chromosome 16q13 and is composed of 26 exons that three alternatively spliced mRNAs that encoded three distinct protein isoforms of very similar size (isoform 1 is 1030 amino acids, isoform 2 is 1029 amino acids, and isoform 3 is 1021 amino acids).
The NCC is also referred to as the thiazide-sensitive Na+/Cl– cotransporter due to the fact that its activity is inhibited by thiazide diuretics. The NCC is the major aldosterone-sensitive Na+ transporter within the DCT. More than 180 mutations in the SLC12A3 gene have been identified, most of which affect either the structure of the NCC protein or its trafficking to the membrane. Indeed, the most frequent mutations in the SCL12A3 gene are those that result in lack of proper glycosylation of the NCC protein which prevents its trafficking to the membrane or interferes with its ability to transport Na+ and Cl–.
The characteristic phenotype observed in patients with Gitelman syndrome are mild renal sodium wasting and low blood pressure with marginally increased levels of aldosterone. The altered levels of aldosterone likely explain the hypokalemia and metabolic alkalosis presenting with Gitelman syndrome. The symptoms of Gitelman syndrome generally do not appear before the age of 6 with most patients not being diagnosed until adulthood. The most symptoms that lead to a diagnosis are polydipsia, nocturia, fatigue, salt craving, parasthesia (burning or prickling sensations), and muscle weakness, aching, and cramping. The majority of the symptoms of Gitelman syndrome can be easily treated with oral administration of magnesium chloride and potassium chloride and the low blood pressure that is common in the disorder can be dealt with by consumption of salt-rich diets.
Liddle Syndrome
Liddle syndrome is an autosomal dominant form of monogenic hypertension with symptoms manifesting early in life. This disorder was first identified and characterized by Grant Liddle in 1963. In addition to the severe hypertension in this disorder there is an accompanying hypokalemia. The characteristic features of Liddle syndrome are salt-sensitive hypertension, hypokalemia, and metabolic alkalosis with an associated reduction in renin activity and reduced aldosterone secretion. Despite the reduced aldosterone secretion, the symptoms of Liddle syndrome present as a typical primary aldosteronism giving rise to the association of the term pseudoaldosteronism with Liddle syndrome.
The symptoms of Liddle syndrome are due to activating mutations in two of the genes encoding the epithelial Na+ channel, ENaC. ENaC is a heterotrimeric transporter composed of α, β, and γ subunits which are encoded by the SCNN1A, SCNN1B, and SCNN1G genes, respectively. The SCNN1A gene is located on chromosome 12p13.31 and is composed of 14 exons that generate three alternatively spliced mRNAs each encoding a distinct protein isoform. The SCNN1B gene is located on chromosome 16p12.2 (very near the SCNN1G gene) and is composed of 17 exons that encode a 640 amino acid protein. The SCNN1G gene is located on chromosome 16p12.2 (very near the SCNN1B gene) and is composed of 13 exons that encode a 649 amino acid protein.
Mutations in the SCNN1B and SCNN1G genes are the causes of Liddle syndrome with mutations in the SCNN1B gene being identified in the individuals in the original pedigree of Liddle syndrome. The mutations in the SCNN1B and SCNN1G genes that result in Liddle syndrome all reside with the cytoplasmic tails of the encoded proteins. The common treatments for Liddle syndrome include the use of the potassium-sparing diuretics amiloride or triamterene. Although there is hypokalemia in Liddle syndrome the use of the aldosterone antagonist, spironolactone, has no therapeutic benefit due to the fact that aldosterone levels are normal to low. Mutations in the SCNN1B gene are associated with another syndrome called pseudohypoaldosteronism type 1 (PHA1).
Gordon Syndrome
Gordon syndrome is a rare autosomal dominant form of hypertension that was first characterized in 1964 by Richard Gordon. Gordon syndrome is a unique form of inherited hypertension in that it is associated with hyperkalemia, whereas all other syndromic forms of hypertension are associated with hypokalemia. Indeed, syndromic hypertension associated with hyperkalemia is diagnostic of Gordon syndrome. The hyperkalemia associated with Gordon syndrome can be severe enough to cause paralytic tetany. Because of the hyperkalemia and risk for tetany associated with this disease it has led to an alternate name termed familial hyperkalemia and hypertension, FHHt.
Patients with Gordon syndrome typically have suppressed plasma renin levels accompanied with low aldosterone levels. It wasn’t until 2001 that the underlying genetic cause of Gordon syndrome was elucidated. The disorder results from mutations in either of two members of the WNK [With No (K)lysine] kinases, WNK1 and WNK4. The functions of the WNK kinases in renal tubular transport processes are described above. The salt-loading hypertension of Gordon syndrome is due to the fact that WNK1 and WNK4 normally inhibit the activity of the Na+/Cl– cotransporter, NCC by preventing its trafficking to the apical membranes of DCT epithelial cells. Therefore, loss of WNK kinase activity results in unregulated NCC presentation in the membrane leading to excess Na+ reabsorption. Given that water generally follows Na+ this leads to increased fluid volume in the vasculature resulting in the hypertension seen in Gordon syndrome.
The hyperkalemia associated with Gordon syndrome is due to the fact that WNK1 and WNK4 normally activate the renal outer medullary K+ channel (ROMK, encoded by the KCNJ1 gene) present in the apical membranes of late distal convoluted tubule (DCT2) cells and connecting tubule cells. The normal function of ROMK is K+ efflux to the tubular lumen. Thus, loss of WNK kinase activity results in significantly impaired K+ secretion accounting for the hyperkalemia associated with the hypertension in Gordon syndrome.
Dent Disease
Dent disease is a term often used to describe a family of related kidney diseases called X-linked recessive nephrolithiasis with kidney failure, X-linked recessive hypophosphatemic rickets, and idiopathic low-molecular-weight proteinuria. The primary form of Dent disease (approximately 60% of cases) is a rare X-linked disorder associated with defects in proximal tubule function resulting in proteinuria and excess Ca2+ secretion (hypercalciuria) in the urine. The excess urinary Ca2+ results in a high incidence of calcium oxalate renal lithiasis.
There are two primary forms of Dent disease, type 1 and type 2. Type 1 Dent disease results from mutations in a gene encoding a member of the voltage-gated chloride channels, specifically the CLCN5 gene. The CLCN5 gene is located on the X chromosome (Xp11.23) and is composed of 17 exons that generate five alternatively spliced mRNAs that collectively encode four different protein isoforms. The CLCN5 protein is localized to endosomal membranes where it is believed to function in the uptake of albumin by the proximal tubule. The precise mechanism by which mutations in the CLCN5 gene result in the associated hypercalciuria in type 1 Dent disease are not understood.
Type 2 Dent disease is characterized by renal Fanconi syndrome in the absence of significant pathology external to the kidneys. Type 2 Dent disease results from mutations in the ORCL gene that encodes the inositol polyphosphate-5-phosphatase enzyme. The ORCL gene is located on the X chromosome (Xq226.1) and is composed of 29 exons that generate three alternatively spliced mRNAs, each of which encode distinct protein isoforms. The ORCL encoded enzyme functions in the regulation of endosomal membrane trafficking, particularly within the proximal tubule but also in other tissues which accounts for the additional symptoms observed in type 2 patients. These additional symptoms include mild intellectual disability, ocular pathology such as cataracts, and hypotonia. Mutations in the ORCL gene are also associated with another disorder called Lowe syndrome.