Last Updated: February 7, 2025
Introduction to Pompe Disease
Glycogen storage disease type 2 (GSD2) is an autosomal recessive disorder that is more commonly known as Pompe disease or acid maltase deficiency (AMD). This disease was originally referred to as Pompe disease since the Dutch pathologist, Joannes Cassianus Pompe, (published in 1932) made the important observation of a massive accumulation of glycogen within the vacuoles of all tissues in a 7-month-old female who died suddenly from idiopathic hypertrophy of the heart. Through the investigations carried out by the Cori’s (Gerty T. Cori and Carl F. Cori) this disease was classified as glycogen storage disease type 2.
Pompe disease is the most severe of all of the glycogen storage diseases. The excess storage of glycogen in the vacuoles is the consequence of defects in the lysosomal hydrolase, acid α-glucosidase which removes glucose residues from glycogen in the lysosomes.
Molecular Biology of Pompe Disease
Acid α-glucosidase is encoded by the GAA (glucosidase, alpha; acid) gene. The GAA gene is located on chromosome 17q25.3 spanning 20 kb and composed of 21 exons that generate five alternatively spliced mRNAs, each of which encode the same 952 amino acid preproprotein.
As of 2025 a total of 398 pathogenic mutations have been identified in the GAA gene causing Pompe disease.
Pompe disease has been shown to be caused by missense, nonsense, and splice-site mutations, as well as by partial deletions and insertions. Some mutations are specific to certain ethnic groups. There are three common allelic forms of acid α-glucosidase that segregate in the general population. These forms are designated GAA1, GAA2, and GAA4.
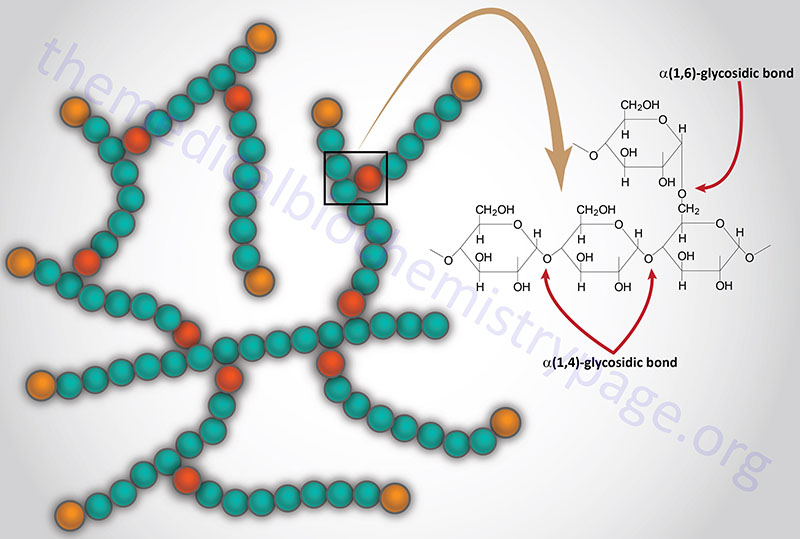
The normal function of acid α-glucosidase is to hydrolyze both α-1,4- and α-1,6-glucosidic linkages at acid pH. The activity of the enzyme leads to the complete hydrolysis of glycogen which is its natural substrate. As would be expected from this activity, deficiency in acid α-glucosidase leads to the accumulation of structurally normal glycogen in the lysosomes of numerous tissues, most notably in cardiac and skeletal muscle.
Clinical Features of Pompe Disease
The clinical presentation of Pompe disease encompasses a wide range of phenotypes but all include various degrees of cardiomegaly. Additional clinical manifestations associated with idiopathic cardiomegaly accompanied by storage of glycogen are indicative of Pompe disease.
The different phenotypes can be classified dependent upon age of onset, extent of organ involvement and the rate of progression to death. Infantile-onset Pompe disease (IOPD) is the most severe and was the phenotype described by JC Pompe. The symptoms of IOPD included hepatomegaly, marked hypotonia, muscular weakness, and death before 1 year of age.
The infantile onset form of Pompe disease presents in the first few months of life. Symptoms include marked cardiomegaly, striking hypotonia (leading to the designation of “floppy baby syndrome”) and rapid progressive muscle weakness. Patients will usually exhibit difficulty with feeding and have respiratory problems that are frequently complicated by pulmonary infection. The prominent cardiomegaly that can be seen on chest X-ray is normally the first indication leading to a preliminary diagnosis of Pompe disease. There is currently no cure for the infantile form of Pompe disease.
Although Pompe disease was originally identified in an infant, IOPD only represents about 25% of all cases. The remaining 75% of cases are classified as late-onset Pompe disease (LOPD). LOPD was originally described in 1969 by Andrew Engel.
Late-onset Pompe disease is a slowly progressing, generally adult onset, proximal myopathic disease. The late onset disease usually presents as late as the second to sixth decade of life, although it can manifest at younger ages, and usually only involves the skeletal muscles. LOPD presents with a pattern of symmetric limb-girdle muscle weakness.
A diagnosis of IOPD versus LOPD is generally accomplished by analysis for the level and activity of acid α-glucosidase in muscle biopsies. Patients with IOPD have less than 1% residual acid α-glucosidase activity, whereas LOPD patients are defined by having only partial deficiency with around 30% of residual acid α-glucosidase activity.
Treatment of Pompe Disease
In 2006 the US FDA approved the use of Myozyme® (alglucosidase alpha) as an enzyme replacement therapy (ERT) for treatment of infantile-onset Pompe disease. Supportive therapy with attention to treatment of respiratory function can impact the course of the disease in the late-onset form. Long term intervention of Pompe disease with alglucosidase alpha has significant limitations. Whereas, lung function improves in the first few months of treatment, there is a gradually return to baseline at 36 months of treatment and a slight decline after 36 months. Walking distances also show improvement in the first three years of treatment but after three years patients experience a decline and by the 10-year assessment walking distances are less than at the start of treatment.
In 2021 a new form of therapy for late-onset Pompe disease (LOPD) patients was approved by the US FDA. This new therapy is sold under the tradename of Nexviazyme (avalglucosidase alfa-ngpt). Avalglucosidase alfa-ngpt is a hydrolytic lysosomal glycogen-specific recombinant human α-glucosidase enzyme that has been conjugated with multiple synthetic bis-mannose-6-phosphate (bis-M6P)-tetra-mannose glycans. This compound contains approximately 15 moles of M6P per mole of enzyme and these mediate the binding of the compound to mannose-6-phosphate receptors and thus, mediates binding to the cell surface.