Last Updated: November 10, 2025
Plant-Derived Phenolic Compounds
Bioactive compounds that are found in plants are referred to as phytochemicals. There is a large array of phytochemicals that have been studied for their clinical benefit in humans with many showing promise as anti-cancer agents. These anti-cancer compounds have been shown to possess chemo-preventive properties (i.e. antimutagenic and anticarcinogenic) as well as being able to interfere with tumor promotion and progression.
The anticancer properties of plants should not seem surprising given that numerous studies have shown that a diet high in fruits, vegetables, and whole grains is strongly associated with a reduced risk of cancer. The US National Institutes of Health (NIH) has identified at least 40 edible plants that possess cancer preventive properties. Within the realm of Chinese herbal medicine there are over 400 species of plants and herbs that are associated with cancer prevention. Estimates place the number of biologically active phytochemicals found in fruits, vegetables, grains, and other plant species at over 5,000.
Among the clinically useful phytochemicals are the vitamins, carotenoids, alkaloids, nitrogen-containing compounds, organosulfur compounds, and the phenolic compounds. Plant-derived phenolic compounds exert a wide variety of biological activities that include antioxidant, anticancer, anti-aging, anti-inflammatory, anti-atherosclerotic, and antiviral properties. Within the plant itself, phenolic compounds are necessary for reproduction, growth, and as defense mechanisms against parasites, predators, and pathogens. Although considered of lesser significance, phenolic compounds also impart the color of plants.
There are literally hundreds of phenolic compounds that have been identified or tested for medicinal benefit. These compounds include non-flavonoid phenolic acids and phenolic acid analogs, stilbenes, curcuminoids, coumarins, lignans, tannins, quinones, and the flavonoids. Phenolic compounds are so called because their chemical structure is composed of one or more aromatic rings containing one or more hydroxyl groups. The physiological and pharmacological functions associated with plant-derived phenolic compounds likely are related to their antioxidant and free radical scavenging properties. The more hydroxyl groups present in a given compound, the more antioxidant is the compound.
Non-Flavonoid Polyphenols
The phenolic acids represent a major class of plant-derived phenolic compounds. The predominant phenolic acids include the hydroxycinnamic acids and the hydroxybenzoic acids. The hydroxycinnamic acids include ferulic acid, caffeic acid, para-coumaric acid (p-coumaric), chlorogenic acid, and sinapic acid. The hydoxybenzoic acids include gallic acid, vanillic acid, p-hydroxybenzoic acid, syringic acid, and protocatechuic acid.
Structurally related polyphenols that are considered members of the phenolic acid analog family include capsaicin, rosmarinic acid, tyrosol, hydroxytyrosol (these latter two compounds are high in white wines), gingerol (responsible for the spicy taste of ginger), gossypol, ellagic acid, cynarin, paradol, and salvianolic acid B. The naturally occurring phenolic acids are found free or conjugated, where the most common conjugation is to a carbohydrate.
Capsaicin
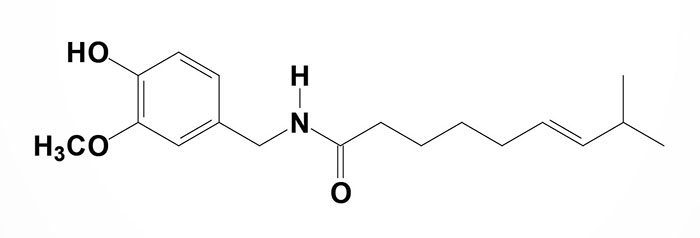
Capsaicin (chemical name is 8-methyl-N-vanillyl-6-nonenamide) is the active compound in chili peppers. The compound is an irritant and causes a burning sensation when contacting any mucous membrane or tissue such as the skin. Cold milk can relieve the burning sensation caused by capsaicin because the milk protein, casein, has a detergent effect on the compound. Current medicinal uses for capsaicin include its use in topical creams for the relief of the itching and inflammation associated with psoriasis. Topical ointments with capsaicin are also used to treat the pain associated with peripheral neuropathy such as that experienced by patients suffering from shingles. Capsaicin has been reported to reduce the digestion of carbohydrates and thus may be useful in the regulation of blood glucose levels in diabetics.
Curcuminoids
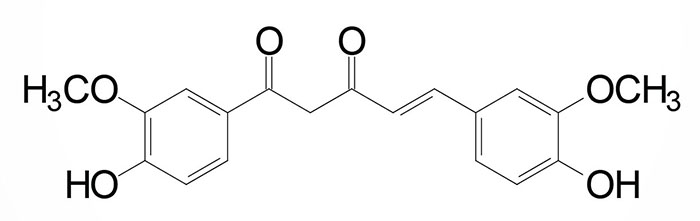
The curcuminoid compounds are derivatives of ferulic acid and are composed of two molecules of ferulic acid linked together. There are three main curcuminoids: curcumin, demethoxycucumin, and bisdemethoxycurcumin (the structure of curcumin is shown below). These compounds are yellow and as such impart their color to spices such as turmeric and mustard. Curcuminoids are found in the Curcuma and Zingiber species of plants that serve as sources of spices such as turmeric and ginger, respectively.
The curcuminoid compounds have been shown to possess antioxidant, anti-aging, anti-inflammatory, antithrombotic, antifibrosis, antimicrobial, anti-parasitic, antiviral, anticarcinogenic, antimutagenic, and hepatoprotective properties. Curcumin is the most well studied of this class of compound.
Curcumin (chemical name diferuloylmethane) is the yellow compound found in the spice turmeric. Turmeric is derived from the rhizomes, the horizontal stems of a plant found underground (e.g. ginger root), of the perennial herb, Curcuma longa Linn, a member of the ginger family (Zingerberaceae).
When curcumin is eaten very little is actually absorbed from the gut. In studies where from 2 to 10 grams of curcumin were eaten alone (i.e. without other foods) there was undetectable to very low levels of the compound detected in the serum. When in the gut, curcumin is unstable and the traces that do pass through the gut are taken up by the liver and rapidly degraded or are conjugated to glucuronic acid and subsequently excreted. Glucuronidation is a typical means by which the liver detoxifies lipid soluble compounds, making them soluble and easily excretable.
Curcumin has been shown to suppress tumor promotion and proliferation, inflammatory signaling, and angiogenesis (the development of new blood vessels). It should be noted that solid tumors cannot grow unless they can promote the development of new blood vessels to bring oxygen-rich blood to the cancerous tissue. Therefore, the antiangiogenic properties of curcumin could play a significant part in its anticancer activity. The anti-inflammatory activity of curcumin is, in part, due to its ability to inhibit enzymes that are necessary for the synthesis of lipid mediators of inflammation. In particular, curcumin inhibits cyclooxygenase-2 (COX-2: this is the same enzyme that is inhibited by the NSAID drug celecoxib) and lipoxygenase.
Curcumin also inhibits inflammatory responses initiated by various stimuli that result in the activation of white blood cells such as macrophages and T-cells, both of which are potent inflammatory mediators. In studies on the effects of curcumin using human cells in culture it has been shown that the compound blocks the release of inducible nitric oxide synthase (iNOS) and COX-2 from airway epithelial cells, prevents COX-2 expression in mammary epithelial cells, inhibits cytokine secretion from macrophages, and blocks the release of cytokines and ROS from arterial cells. Curcumin also exerts cytoprotective effects that enhance cellular survival. Much of this activity is due to the antioxidant properties of curcumin.
Previous in vivo studies have demonstrated that administration of curcumin can lead to decreases in the level of cholesterol in the blood. These effects of curcumin on cholesterol levels were thought to be related to upregulation of LDL receptor. However, since plasma cholesterol levels are also influenced by the uptake of cholesterol in the gut, which is mediated by a specific transporter, Niemann-Pick C1-like 1 (NPC1L1) protein, it is possible that curcumin exerts its cholesterol lowering effects via inhibition of this intestinal cholesterol uptake mechanism. Indeed, in a study using an intestinal cell culture system (Caco-2 cells) it was shown that treatment with curcumin results in a down-regulation of the expression of the NPC1L1 gene resulting in reduced levels of the protein present in the membrane of Caco-2 cells. The NPC1L1 protein is also highly expressed in human liver. The hepatic function of NPC1L1 is presumed to limit excessive biliary cholesterol loss. NPC1L1-dependent sterol uptake is regulated by cellular cholesterol content. Recently studies have shown that NPC1L1 inhibition results in beneficial effects on components of the metabolic syndrome, such as obesity, insulin resistance, and fatty liver, in addition to atherosclerosis. Therefore, consumption of curcumin may have clinical benefits in the management of the metabolic syndrome and its associated cardiovascular complications.
In patients suffering from inflammatory bowel disease, taking 550mg curcumin twice daily resulted in significant amelioration of inflammatory symptoms. In another study, patients with rheumatoid arthritis took 1220mg daily and experienced a reduction in inflammatory symptoms.
Flavonoid Polyphenols
The flavonoids represent a group of related phenolic compounds of which more than 4000 different types have been identified as naturally occurring in plants. The flavonoids are compounds that, like the vitamins, are not produced by the body and must be acquired from the diet or nutritional supplements. The flavonoids are categorized into the flavones, flavonols, flavanones, flavanonols, flavanols (flavan-3-ols and flavan-3,4-diols), anthocyanins (anthocyanidins), chalcones, isoflavonoids (primarily isoflavones), neoflavonoids, and biflavonoids. These various flavonoid compounds are found in nature either free or conjugated to a sugar (carbohydrate) molecule via a glycosidic linkage. The most common sugars found linked to flavonoids are glucose, galactose, arabinose, glucuronic acid, and rhamnose.
The most common flavones are luteolin, apigenin, and chryslin and their respective glycosides. These compounds are found in broccoli, legumes, cherries, tea, olives, thyme, and parsley. The flavones are also found in medicinal herbs such as the roots of Scutellaria baicalensis (Skullcap that is native to North America), the inflorescences (clustered flowers) of Chrysanthmum morifolium, and the aerial parts of Artemisia annua (Sweet Wormwood, Sweet Annie, Sweet Sagewort or Annual Wormwood).
The common flavonols are quercetin, kaempferol, myricetin, and galagin and their respective glycosides. Quercitrin is the name of the sugar-linked quercetin. The flavonols are found in a range of plants including, onions, broccoli, tomato, kale, buckwheat, cherries, apples, berries, tea, red wine, cumin, and caraway. Flavonols are also found in medicinal herbs from the flowers of Sophora japonica (Japanese pagodatree, scholar-tree) and Rosa chinensis (China rose), the aerial parts of Artemisia annua, the rhizomes of Alpinia officinarum (a plant of the ginger family also known as lesser galangal), and the fruits of Crataegus pinnatifida (Chinese hawthorn).
The flavanones include naringenin, hespercetin, and eridictyol as well as the glycoslylated forms. The glycoside of naringenin is called naringin and that of hespercetin is called hesperidin. The flavanones are found primarily in citrus fruits such as lemons, oranges, and grapes and the medicinal herbs derived from Rutaceae, Rosaceae, and Leguminosae.
Flavanols include catechin, epicatechin, epigallocatechin, epicatechin gallate (ECG), and epigallocatechin gallate (EGCG). These polyphenols are found in apples, berries, tea, and cocoa. Many flavanol compounds have been associated with potential anti-aging properties such as catechin and EGCG.
Anthocyanins, including anthocyanidins (such as cyanidin, delphinidin, malvidin, peonidin, and pelargonidin) are widely distributed in medicinal herbs and dietary plants such as blueberries, bayberries, grape skins, red cabbage, beans, purple sweet potatoes, and red/purple rice and corn.
Isoflavones include genistein, glycitein, daidzein, and their respective glycosides. The sugar-linked genistein is called genistin. These compounds are found in soybeans, legumes, and red clover as well as medicinal herbs from plants of the Leguminosae family and from the roots of Astragalus mongholicus (Milk-Vetch and Huang-qi).
Quercetin
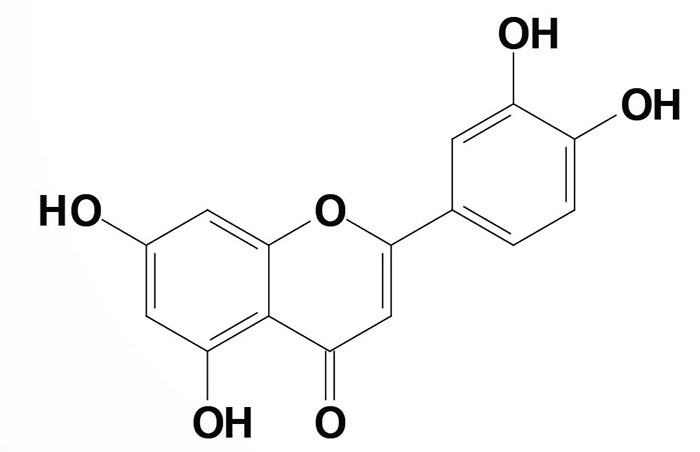
Quercetin [chemical name is 2-(3,4-dihydroxypheny)-3,5,7-trihydroxy-4H-1-benzopyran-4-one] is one of the most potent antioxidant polyphenols which explains its use as a dietary supplement.
Quercetin is found in numerous foods such as brassica vegetables (e.g. broccoli, cauliflower, cabbage, brussel sprouts, bok choy), apples, berries, red onions, citrus fruits, and tea made from Camellia sinensis, as well as many seeds, nuts, leaves, barks, and flowers. Very high concentrations of quercetin are found in capers and lovage (Levisticum officinale, similar in appearance and taste to celery), on the order of 2mg per gram of plant. Quercetin is available in highly purified extracts for sale as a dietary supplement which allow for the consumption of 500–1000mg per day. This is the equivalent of eating 5–10 kilograms (11–22 pounds) of apples each day.
The sugar conjugated quercetin compounds are very hydrophilic (meaning they do not interact with water) and were thought to be poorly absorbed from the gut following consumption. However, evidence shows that around 50% of quercetin glycosides are absorbed versus 25% for the aglycon form (sugar molecule removed). The biochemical basis for this absorption difference is believed to be due to the intestinal uptake process that involves a carrier-mediated transport or a coupled deglycosylation transport mechanism. After uptake by carrier-mediated processes quercetin glycosides have their sugar molecule removed by intracellular glycosidases. Following absorption quercetin is metabolized by the small intestine, colon, liver, and kidney. In animal models of quercetin absorption and tissue distribution, highest concentrations were found in the lung, liver, and kidney. Because the half-life of quercetin in the plasma and tissues is long (on the order of 28 hours), repeated intake with supplements can lead to considerable plasma levels of the compound.
In addition to antioxidant activity (but likely related to this activity) quercetin exhibits anti-inflammatory, antiproliferative, and apoptotic effects both on cells in culture as well as when ingested. In addition to cancer, quercetin is active in many processes of diseases related to ageing such as cardiovascular and neurodegenerative disorders. Many studies have looked at the effects of quercetin in the treatment of breast, ovarian, colon cancers, and leukemias. The anti-tumor properties of quercetin are diverse and include the ability to modulate the metabolism of carcinogens through inhibition and/or induction of enzymes that are involved in their conversion to non-toxic compounds. This latter process is referred to as xenobiotic metabolism. Research into the anti-cancer effects of quercetin have shown that the compound can induce cell cycle arrest and DNA strand breakage resulting in apoptosis.
A gene that is found mutated in numerous types of cancer is called TP53 whose protein product (p53) functions to regulate the progression of cells through the cell cycle. Quercetin has been shown to down regulate the expression of mutant TP53 in breast cancer cells to nearly undetectable levels. The effect of this down regulation is the arrest of cells at the point in the cell cycle prior to cell division. Of significance is the fact that quercetin has a much reduced effect on the expression of the normal TP53 gene.
Quercetin has also been shown, in animal models, to lower blood pressure and ameliorate hyperglycemia and conditions resulting from hyperglycemia. In a trial involving pre-hypertensive and stage 1 hypertensive patients, the consumption of 730mg per day of quercetin for 4 weeks led to a reduction in blood pressure but did not have any effect on the parameters of oxidative stress. Oxidative stress is referred to as an imbalance between the production of, and protection against, reactive oxygen species (ROS) and can result from overproduction of ROS and/or impairment of the endogenous antioxidant defense systems in the body.
Additional activities attributed to quercetin include regulation of caspase-3 (involved in triggering apoptosis), telomerase (an enzyme of DNA replication), lymphocyte tyrosine kinase (kinase are enzymes that add a phosphate moiety to their substrate), and other tyrosine kinases and serine/threonine kinases. Tyrosine, serine, and threonine are amino acids found in proteins. Quercetin increases the activity of superoxide dismutase, catalase, and glutathione peroxidase explaining its powerful antioxidant properties. One major benefit of the increase in these latter enzymes is a significant decrease in the oxidation and peroxidation of membrane lipids, thereby, preventing cell damage.
Naringenin
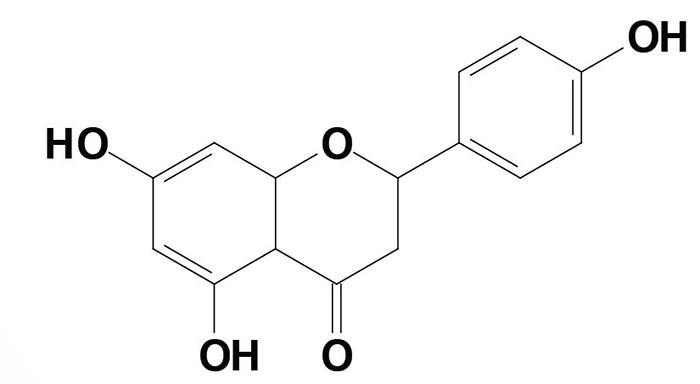
Naringenin is the most abundant citrus-derived flavanone. The tomato is a rich source of the naringenin variant molecule identified as naringenin chalcone. This molecule is structurally similar to naringenin but lacks the –O– of the second aromatic ring.
Naringenin has been shown to exert antitumor, antioxidant, and anti-inflammatory effects. Naringenin is a member of the phytoestrogen family of compounds and as such has been shown to possess estrogenic effects on cells in culture. One important activity demonstrated for naringenin is the activation of two distinct receptors for estrogen termed estrogen receptor-alpha (ERα) and ER-beta (ERβ). The anti-inflammatory effects of naringenin chalcone are the result of inhibition of the synthesis and release of pro-inflammatory cytokines (proteins) from white blood cells (e.g. macrophages).
Although there are now dietary supplements that contain naringenin, there is currently no scientific data to demonstrate the bioavailability of the compound from these supplements nor their benefits in humans.
Genistein
Genistein (chemical name is 4′,5,7-trihydroxyisoflavone) is an isoflavone. Genistein is synthesized in plants from naringenin (see above) by a novel ring migration reaction catalyzed by the cytochrome P450 (CYP) enzyme isoflavone synthase. The compound is a common precursor in the biosynthesis of phytoalexins and phytoanticipins in legumes. Phytoalexins and phytoanticipins are natural antimicrobials synthesized in plants.
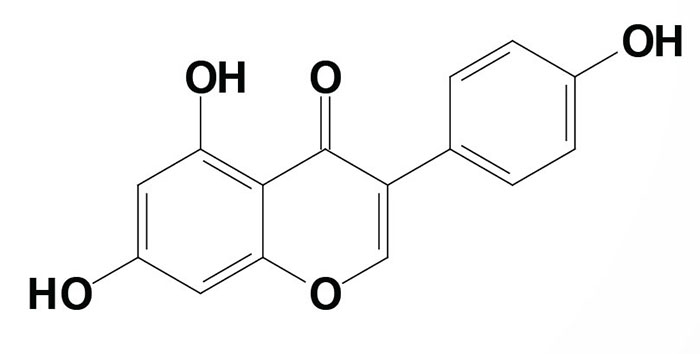
Genistein is also found in soybean seeds and red clover and medicinal herbs such as those prepared from the roots of Astragalus mongholicus. Genistein is a member of a family of molecules called phytoestrogens. Phytoestrogens are plant-derived compounds that exhibit steroid hormone activity similar to estrogen. Genistein has a chemical structure similar to estrogen and it binds to both of the human estrogen receptors (ERα and ERβ) in vitro although at several-fold lower binding affinities: 0.017% ERα and 7.4% ERβ binding for genistein as compared to 100% for both receptors by 17β-estradiol.
The potential chemopreventive and protective effects of genistein have been extensively studied. Genistein accumulates in soy and soy products at concentrations as high as 1.5 mg/g, which depend on factors such as the soy variety, environmental factors during growth and harvest and processing.
Genistein has been shown to possess a wide variety of pharmacological effects in animal cells and animal model studies. These activities include tyrosine kinase inhibition, chemoprevention of breast and prostate cancers, prevention of cardiovascular disease and amelioration of post-menopausal ailments. Despite an extensive literature on the effects of genistein as a dietary supplement, questions still exist as to its potential overall benefits as a component of the human diet.
Genistein has been shown to promote the growth of estrogen-responsive breast and endometrial cancer cells in culture. Similar to the known effect of 17β-estradiol, treatment of breast (T47D and MCF-7) and endometrial (ECC-1) cancer cells with phytoestrogens, such as genistein, induces cell proliferation, cell-cycle progression and transactivation of the estrogen response element (ERE). The effects of genistein on these types of cancer cells can be reversed by treatment with carotenoids such as lycopene, phytoene, and phytofluene (see below). This cancer promoting effect of genistein on estrogen-responsive cancers is not seen in estrogen receptor-negative breast cancer, and in fact in these cell types genistein inhibits cell growth. The inhibitory action of genistein is effected through its ability to inhibit tyrosine kinase activity such as that associated with many cell surface growth factor receptors.
Catechin
Catechin [chemical name is (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol] is a flavanol found predominantly in extracts of the deciduous tree Senegalia catechu (khair or kachu). The catechins can exist in multiple chemical forms (isomers) with two isomers in the trans configuration and two in the cis configuration. The cis isomers are referred to as epicatechins. The (+)-catechin isomer is the most common form.
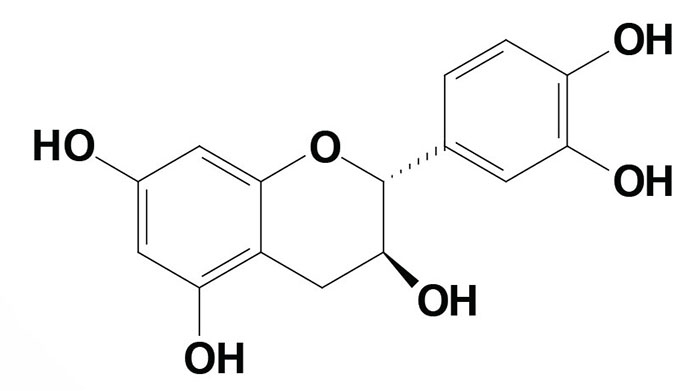
Although there have been a few reports indicating that (+)-catechin possesses antioxidant and anti-aging effects, the major catechin compounds exhibiting these properties are the epicatechins, in particular epigallocatechin and epigallocatechin gallate (EGCG).
Fisetin
Fisetin (chemical name is 3,3′,4′,7-tetrahydroxyflavone) is a naturally occurring flavonoid commonly found in fruits and vegetables.
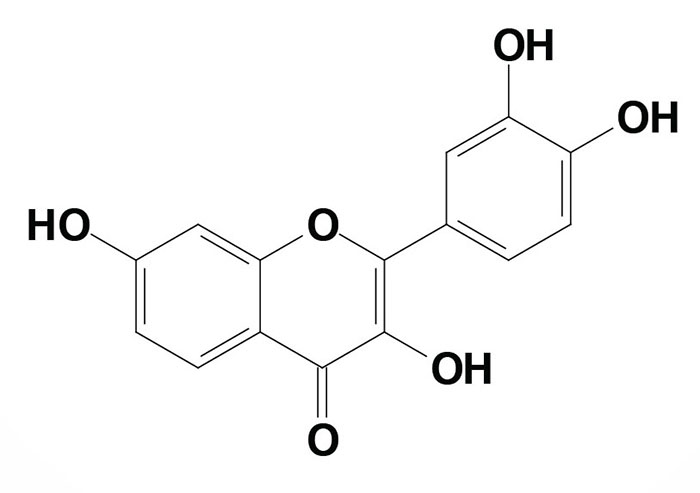
The highest levels of fisetin are found in strawberries with 5-10-fold lower amounts in apples, kiwi fruit, peaches, grapes, persimmons, onions, cucumbers, and tomatoes. Although detected in these plant sources, the level of fisetin bioavailability from them has yet to be studies.
The biological activity of fisetin was discovered in a screen for compounds that could prevent nerve cell death due to oxidative stress. Several flavonids were assayed in these studies and only fisetin and quercetin were found to be effective. Both of these compounds were shown to maintain elevated glutathione (GSH) levels in the presence of oxidative stress. GSH is a tripeptide that serves as potent naturally occurring antioxidant.
In addition to its ability to act directly as an antioxidant as well as indirectly through elevations in GSH levels, fisetin has subsequently been shown enhance mitochondrial function in the presence of conditions of oxidative stress. Additional activities of fisetin include inhibition of the activity of the enzyme 5-lipoxygenase (5-LOX). 5-LOX is involved in the synthesis of pro-inflammatory lipid peroxides and thus, inhibition of its activity by fisetin exerts an anti-inflammatory effect. In addition, fisetin has demonstrated differentiation effects as shown by its ability to induce the neuronal differentiation of PC12 cells (a rat adrenal medullary cancer cell line).
Given the ability of fisetin to act as an anti-oxidant and to promote nerve cell differentiation, proliferation, and protection it is suggested that this flavonoid may be useful in the treatment and/or prevention of age-related cognitive decline in brain function. One way to examine the effect of a substance on learning and memory (cognitive functions) is to utilize animal models of long-term potentiation (LTP). LTP represents a long-lasting enhancement in signal transmission between two neurons resulting from synchronous stimulation. Since memories are thought to be encoded by modification of synaptic strength, LTP is generally considered to be one of the major cellular mechanisms that underlies the processes of learning and memory. In rat studies the administration of fisetin has been shown to induce LTP in a dose-dependent manner and to last for up to 60 minutes after fisetin administration. Inhibition of specific signal transduction processes interfere with the activity of fisetin demonstrating that the compound functions in LTP induction via a defined activation pathway. The specifics of this pathway are beyond the scope of this overview of fisetin. In addition to enhancing LTP in rat, administration of fisetin to mice increases their object recognition capability, a measure of memory.
Given that flavonoids, such as fisetin, are extensively metabolized following injection or ingestion it has been argued that the levels of these compounds, in the brain, following administration may not be sufficient to exert positive effects. However, research has shown that fisetin exhibits a high brain uptake potential and that following intraperitoneal injection fisetin can be detected in brains of rats. Coupled with brain detection is a significant reduction in cerebral damage in these rats in an experimental model of stroke.
Malvidin
Malvidin [chemical name: 3,5,7-trihydroxy-2-(4-hydroxy- 3,5-dimethoxyphenyl) chromenium] is an O-methylated anthocyanidin that imparts the blue color to the petals of flowers from plants of the Primula genus. Common plants in this family are primrose (Primula vulgaris), cowslip (Primula veris), and oxlip (Primula elatior). Malvidin also contributes to the color of red wine where Vitis vinifera (common grape vine) is its major source.
Malvidin has been shown to function as an antioxidant by increasing the levels of the antioxidant enzyme, superoxide dismutase resulting in reduced levels of reactive oxygen species (ROS) in cells in culture. Enhanced reductions in ROS likely contribute to the potential for malvidin to function in an anti-aging capacity. However, no clinical data in humans is available to verify claims that malvidin can exert anti-aging effects.
Carotenoid Antioxidants
All carotenoids are derivatives of tetraterpenes, meaning that they are produced from 8 isoprene molecules and contain 40 carbon atoms. The carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, and fungi. There are over 1,100 known carotenoids which can be further categorized into two classes identified as the carotenes and the xanthophylls. The xanthophylls contain oxygen, whereas the carotenes do not.
Carotenoids that contain unsubstituted beta-ionone rings, which includes β-carotene, α-carotene, β-cryptoxanthin, and γ-carotene possess vitamin A activity which means they can be converted to retinol. In the eye, lutein, meso-zeaxanthin, and zeaxanthin are present as macular pigments whose importance in visual function.
Astaxanthin
Astaxanthin is a member of the xanthophyll (“yellow leaves”) family of carotenoids. It belongs to a larger class of phytochemicals known as terpenes. Like many carotenoids, astaxanthin is a colorful, lipid-soluble pigment. Astaxanthin is produced by microalgae, yeast, salmon, trout, krill, shrimp, crayfish, and crustaceans. Astaxanthin is an essential nutritional component for adequate growth and reproduction in these organisms.
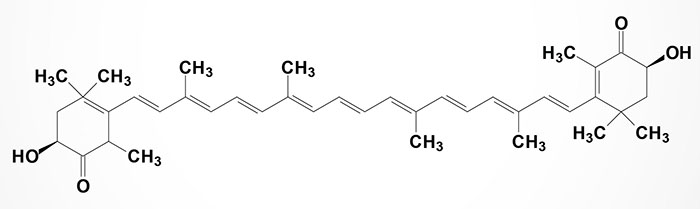
Astaxanthin, unlike some carotenoids, does not convert to vitamin A (retinol) in the human body. Too much vitamin A is toxic for a human, but astaxanthin is not. Depending on the source, astaxanthin can be found free or in association with other compounds. It may be esterified at one or both hydroxyl groups with different fatty acids such as palmitic, oleic, stearic, or linoleic acids. It can also form a chemical complex with proteins (carotenoproteins) or lipoproteins (carotenolipoproteins).
One of the most important properties of astaxanthin is its antioxidant properties which have been reported to surpass those of β-carotene (from which vitamin A is derived) or even α-tocopherol (vitamin E). It is claimed to possess as much as 10 times the antioxidant potential of other carotenoids such as zeaxanthin, lutein, canthaxanthin, and β-carotene; and 100 times more that α-tocopherol. However, other sources suggest astaxanthin has slightly lower antioxidant activity than other carotenoids. Thus, caution must be observed when studying and comparing the antioxidant activity of the various carotenoids since results will be dependent on the experimental conditions under which the assays are performed. In spite of the variability in assessed antioxidant potential, the antioxidant activity of astaxanthin has been attributed with the potential to protect against a wide range of ailments such as cardiovascular disease, numerous types of cancer and some diseases of the immune system.
The potential activity of carotenoids, such as astaxanthin, against cancer has been the focus of much research due to the association between low levels of these compounds in the body and increased prevalence of cancer. Research in mice and rats has shown that oral administration of astaxanthin inhibits carcinogenesis in mice urinary bladder, in the oral cavity, and rat colon. These effects have been partially attributed to suppression of cell proliferation. In another study where mice were inoculated with fibrosarcoma cells, the dietary administration of astaxanthin suppressed tumor growth and stimulated the immune response against the antigen which expresses the tumor. Mice fed a diet containing 0, 0.1% and 0.4% astaxanthin, β-carotene or canthaxanthin during three weeks before inoculating the mammary fat pad with tumor cells demonstrated that the level of growth inhibition of the tumor cells by astaxanthin was dependent on the dose and more effective than the other two carotenoids tested.
Data has also demonstrated that astaxanthin promotes immune responses, by inhibiting lipid peroxidation, that in turn attenuate liver metastasis induced by stress in mice. Additionally, the effects of astaxanthin and other carotenoids on proliferation of human breast cancer cells (MCF-7) have also been examined. In one study it was shown that β-carotene and lycopene were more effective than astaxanthin at inhibiting the proliferation of MCF-7 cells in culture.
Phytoene and Phytofluene
Phytoene and phytofluene are colorless carotenoids found in abundance in tomatoes, carrots, apricots, oranges, watermelons, and peppers. These carotenoids are also present in numerous microalga such as Dunaliella bardawil, Dunaliella salina and Chlorella sorokiniana.
Phytoene and phytofluene have been implicated in a reduction in breast, endometrial, and prostate cancer risk. Phytofluene accumulates to the highest levels in the liver whereas lycopene is observed at highest levels in the prostate. Although there is a specificity to tissue distribution, all tissues except the adrenal glands, accumulate some level of phytoene, phytofluene, and lycopene following ingestion of tomato extracts.
Like other carotenoids, phytoene and phytofluene have antioxidant activity. Of potential significance to the prevention and treatment of cardiovascular disease, both of these carotenoids can inhibit the oxidation of low-density lipoproteins (LDL) to the same degree as β-carotene and α-tocopherol.
Phytoene is synthesized from geranylgeranyl pyrophosphate via the action of the enzyme phytoene synthase. Phytoene then serves as the precursor for a number of other carotenoids, including lycopene. Through the actions of numerous enzymes phytoene is also converted to α-carotene, β-carotene, lutein, and zeaxanthin.
Recent evidence, using phytoene and phytoene-rich extracts from microalgae, has found that phytoene exhibits anti-aging characteristics in the model organism, the round worm Caenorhabditis elegans.
Lutein and Zeaxanthin
Lutein and zeaxanthin are members of the xanthophyll family of carotenoids. They belong to a larger class of phytochemicals known as terpenes. These carotenoids contain 40 carbon atoms with hydroxylated cyclic structures at each end. Lutein is structurally similar to zeaxanthin with the only difference between these two carotenoids being the location of one of the carbon-carbon double bonds in one of the hydroxylated cyclic structures. The name zeaxanthin is derived from Zea mays (common yellow maize corn) in which zeaxanthin provides the primary yellow pigment, plus the Greek word for yellow.
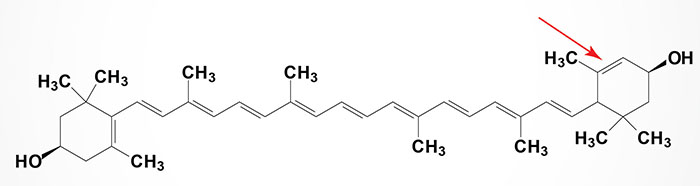
Lutein and zeaxanthin are present in a wide variety of plant sources, such as leafy green vegetables (kale, turnip, and spinach etc.) as well as a few animal sources, such as egg yolk. These molecules are also high in liver, adrenal glands, adipose tissue, pancreas, kidney, and breast, whereas lower levels have been reported in the lung, spleen, heart, testes, thyroid, ovary, and skin. Adipose tissue and retina may compete for uptake of lutein and zeaxanthin. When compared with the retina, adipose tissue preferentially takes up lutein. It is estimated that more than 80% of the total number of carotenoids in the body are found in adipose tissue, which may serve as a reservoir for these compounds.
Lutein, along with zeaxanthin, are the two carotenoids contained within the retina of the eye. Within the central macula, zeaxanthin is the dominant component, whereas in the peripheral retina, lutein predominates. Because both lutein and zeaxanthin are enriched in the macular region of the eye they are referred to as macular pigment (MP). Because neither carotenoid can be synthesized in the human body they must be supplied in the diet. Both lutein and zeaxanthin are essential in the protection of the retina from oxidative damage. Lutein and zeaxanthin selectively bind to tubulin, a structural protein that helps form the cytoskeleton in cone axons, possibly maintaining the structural integrity and improving visual function. Tubulin is abundant in the retina, which may explain the selective accumulation of lutein and zeaxanthin in the macula in comparison to other carotenoids.
Following ingestion lutein and zeaxanthin are absorbed by the enterocytes of the intestinal mucosa and are then transported in chylomicrons to the liver. Low- and high-density lipoproteins (LDL, HDL) transport lutein and zeaxanthin to various tissues. HDL carries primarily lutein and zeaxanthin whereas LDL transports other carotenoids in addition to lutein and zeaxanthin.
The retina is particularly susceptible to oxidative stress because it is highly vascularized in order to provide the visual cells the large amount of oxygen they need for aerobic metabolism. Retinal cells are also enriched in polyunsaturated fatty acids (PUFA) which are susceptible to oxidative damage because their conjugated double bonds are sources of hydrogen atoms. The rod cell outer segments have a high concentration of long-chain PUFA accounting for approximately 50% of the lipid bilayer. Docosahexaenoic acid (DHA) makes up approximately 50% of the phospholipid content of rod cells.
The retina is also rich in antioxidant enzymes and has a high capacity for scavenging free radicals. Reactive oxygen species (ROS), such as free radicals, hydrogen peroxide, and singlet oxygen, are byproducts of oxygen metabolism. Lutein and zeaxanthin have polar end groups that protrude from the lipid cell membrane and interact with ROS outside the membrane making them effective antioxidants by reducing the amount of short-wave light reaching the photoreceptor outer segments. Light damage to the retina may stimulate peroxidation of PUFA in the membrane, which may result in loss of membrane function and structural integrity. Evidence shows protective effects of lutein and zeaxanthin against uv-induced oxidative damage, lipid peroxidation, quenching singlet oxygen, reducing inflammatory response, and filtering blue light.
It is these effects of lutein and zeaxanthin that indicate their utility as dietary supplements in the protection against age-related macular degeneration (AMD). In a case-controlled study it was found that a significant relationship exists between a reduction in risk for AMD with increased amounts of lutein and zeaxanthin in the retinas when donors with AMD were compared to those without at autopsy. In individuals with the highest levels of lutein and zeaxanthin there was a correlated 82% lower risk for AMD compared to those individuals having the lowest detectable levels. A similar study found that autopsy eyes had 30% lower concentrations of lutein and zeaxanthin in AMD retinas compared to controls as well as reduced carotenoid levels throughout the retina.
Lycopene
Lycopene is a carotenoid but does not exhibit any vitamin A activity. The synthesis of lycopene involves a pathway that begins with another carotenoid, phytoene.
Lycopene imparts a red color to foods such as tomatoes, red carrots, watermelon, and papaya. Because of its structural similarity to beta-carotene (from which vitamin A is derived), lycopene is a potent antioxidant.
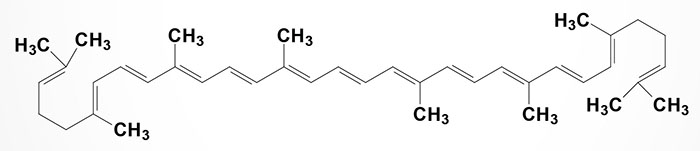
Lycopene has been shown to exert a chemopreventive effect against prostate cancer but its role in prostate cancer progression is unknown. In addition to its effects on prostate cancer cells, lycopene exerts anticancer effects on lung, colon, stomach, and skin cancer cells in culture. In a recent study of men with prostate cancer lycopene was shown to reduce the plasma levels of prostate-specific antigen (PSA) and to lower urinary tract symptoms and pain.
In studies on the anticancer activities of lycopene it has been shown that this carotenoid can interfere with the estrogenic and cancer promoting effects of genistein on human breast and endometrial cancer cells. Lycopene (as well as phytoene, phytofluene, and β-carotene) can inhibit cancer cell proliferation induced by either 17β-estradiol or genistein. The inhibition of cell growth by lycopene was accompanied by reduction in the rate of cell-cycle progression from G1 to S phase. In addition, lycopene (as well as phytoene, phytofluene, and β-carotene) inhibited estrogen-induced transactivation of genes containing an estrogen response element (ERE). This inhibition of gene activation was observed at estrogen target genes that are activated by both estrogen receptors (ER), ERα and ERβ.
Phytoene and Phytofluene
Phytoene and phytofluene are colorless carotenoids found in abundance in tomatoes, carrots, apricots, oranges, watermelons, and peppers. These carotenoids are also present in numerous microalga such as Dunaliella bardawil and Dunaliella salina.
Phytoene and phytofluene have been implicated in a reduction in breast, endometrial, and prostate cancer risk. Phytofluene accumulates to the highest levels in the liver whereas lycopene is observed at highest levels in the prostate. Although there is a specificity to tissue distribution, all tissues except the adrenal glands, accumulate some level of phytoene, phytofluene, and lycopene following ingestion of tomato extracts.
Like other carotenoids, phytoene and phytofluene have antioxidant activity. Of potential significance to the prevention and treatment of cardiovascular disease, both of these carotenoids can inhibit the oxidation of low-density lipoproteins (LDL) to the same degree as β-carotene and α-tocopherol.
Phytoene is synthesized from geranylgeranyl pyrophosphate via the action of the enzyme phytoene synthase. Phytoene then serves as the precursor for a number of other carotenoids, including lycopene. Through the actions of numerous enzymes phytoene is converted to α- and β-carotene, lutein, and zeaxanthin.
Resveratrol
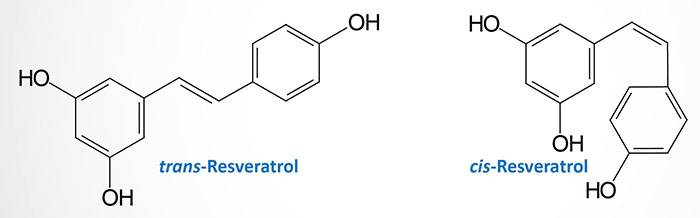
Resveratrol is a chemical compound that is a member of a family of polyphenols called viniferins. The compound was first isolated from the roots of Veratrum grandiflorum (white hellebore). The chemical name for resveratrol is trans-3,4,5′-trihydroxystilbene (or also 3,4′,5-stilbenetriol).
Resveratrol and related types of polyphenols are antioxidants that are enriched in grapes and red wines. The Vitis vinifera (common grape vine), Vitis labrusca (Fox grape), and Vitis rotundifolia (muscadine) grapes contain high concentrations of resveratrol. Vitis vinfera is commonly found in the Mediterranean region, central Europe, and southwestern Asia from Morocco and Spain north to southern Germany and east to northern Iran. Vitis labrusca is native to the eastern United States and is the source of Concord grapes, as well as Catawba grapes, Niagara grapes, and Delaware grapes. Vitis rotundifolia is native to the southeastern United States and is cultivated for wine, juice and jelly production.
Resveratrol is also found in other fruits such as strawberries, raspberries, blueberries, mulberries, cranberries, bilberries, lingo berries, sparkle-berries, jackfruit, deer berries, and partridge berries. Resveratrol is also found in the leaves and flowers of many other plants including peanut root, eucalyptus, spruce, lily, butterfly orchid, corn lily, white hellebore, Scots pine, Eastern white pine, and Japanese knotweed.
Resveratrol is also a phytoalexin (“defender of the plant”). Phytoalexins are antimicrobial substances synthesized de novo by plants that accumulate rapidly at areas of pathogen infection. Resveratrol is produced in plants via the action of the enzyme stilbene synthase not only in response to pathogen invasion but also in response to ozone exposure, heavy metals, sunlight, and changes in climate. Resveratrol exists in nature in both the trans- and cis-stereoisomeric forms with heat and ultraviolet radiation inducing the trans– to cis– isomerization. Both the cis– and the trans– forms of resveratrol exhibit the same level of biological activity. However, in studies on the biological effects of resveratrol it is the trans– form that is most used.
The range of action of resveratrol is broad. This compound has been shown to exert anti-inflammatory, anti-carcinogenic, anti-tumorigenic, and anti-aging effect. In addition, resveratrol inhibits platelet aggregation, a process required for blood coagulation, and as such plays a significant role in the cardioprotective activities of the compound. Resveratrol also acts as a phytoestrogen. Phytoestrogens are plant-derived compounds that can either mimic or inhibit the female sex hormone, estrogen.
Resveratrol has also been shown to ameliorate metabolic defects that occur as a consequence of normal ageing processes. These “age restricting” effects on metabolism, exerted by resveratrol, are the result of its ability to inhibit a class of enzymes known as phosphodiesterases. These enzymes normally degrade the intracellular “second messenger” cAMP. Therefore, the action of resveratrol results in elevated cAMP levels with concomitant increases in events downstream of this signaling molecule. One important effect is the prevention of diet-induced obesity, increased lipid oxidation due to enhanced mitochondrial function, and increased glucose tolerance.
There are multiple intracellular targets of resveratrol which lead to alterations in cell growth, inflammation, apoptosis (programmed cell death), angiogenesis (the growth of new blood vessels), and metastasis and invasion of cancer cells. Perhaps the most striking effect of resveratrol is the ability of this compound to induce the processes that result in longevity in various model organisms (and presumably humans). The longevity-inducing effects of resveratrol are similar to those that result from calorie restriction. Many of the activities of resveratrol are of an anti-oxidant nature. However, increasing evidence demonstrates that resveratrol also exhibits pro-oxidant activities that result in oxidative DNA damage leading to apoptosis and cell death. This latter activity is related to the potential anti-cancer mechanisms of resveratrol.
Fungal infections in grape plants occur with higher frequency in areas of cool climate. Thus, grapes grown in regions of cool climate have high concentrations of resveratrol. However, since ultraviolet radiation from the sun also induces the synthesis of resveratrol, grapes grown in equatorial regions also have high concentrations of resveratrol. Red wines of differing origins contain from 0–18 micrograms per milliliter (μg/ml) of trans-resveratrol with the level of cis-resveratrol ranging from 0–5μg/ml. Muscadine grapes can contain up to 40 times more resveratrol than common grapes. Examples of the differences in resveratrol content in red wines can be seen in a comparison of Brazilian red wines which contain 18μg/ml, Australian Pinot Noir with 13μg/ml, and Swiss reds that contain only 2–3μg/ml. In addition, depending upon the location the same kind of grapevine yields significantly different levels of resveratrol in the wines produced. The Cabernet Sauvignon wines produced in Trentino, Italy contain up to 7μg/ml, whereas the same wines from Napa Valley in California yield only 0.09μg/ml.
Also, although the major difference between red wines and white wines is that in the production process the grape skins are removed in the making of white wines, there are still antioxidant compounds in white wines. However, the level of resveratrol in white wines is low. The major antioxidant compounds in white wines are tyrosol and hydroxytyrosol both of which can induce the anti-aging pathways that are activated by resveratrol.
Of potential clinical significance is the difference in bioavailability of resveratrol obtained from different preparations of grape products. Whereas, the resveratrol content of grape juice can be very high, the concentration of trans-resveratrol found in the blood after grape juice consumption is negligible. This latter fact is related to the absence of ethanol (alcohol) in grape juices and other non-wine sources of resveratrol. When resveratrol is conjugated to compounds that increase its water solubility and thus, presumably its bioavailability, high levels of resveratrol are still not observed in the blood. This is due to the fact that most of the conjugated compound is excreted in the urine.
It is also important to note that there are hundreds of antioxidant compounds in the skin and seeds of grapes and that resveratrol is not the only beneficial polyphenolic compound. The polyphenolic antioxidant compounds in grape skins and seeds consist of the flavonoids and the nonflavonoids. Resveratrol belongs to the nonflavonoid class which also includes the hydroxycinnamates (caffeic, caftaric, and coutaric acids), and the hydroxybenzoates. The polyphenolic flavonoids consist of the flavonols (quercetin and myricetin), flavanols or flavan-3-ols (catechin and epicatechin), and anthocyanins.
Resveratrol consumption is associated with a reduced risk of cardiovascular, cerebrovascular, and peripheral vascular disease. The cardioprotective effects of resveratrol are exerted at relatively low doses in the range of 2.5–5.6 milligrams per kilogram of body weight per day (mg/kg/day). The beneficial effects of resveratrol on the vascular system are many. One major effect of resveratrol in the blood is the prevention of oxidation of low density lipoproteins (LDL). Oxidized LDL contributes significantly to the development of atherosclerosis (fatty plaques in the vessels).
Additionally, resveratrol exhibits an ability to reduce platelet and monocyte (a type of white blood cell) adhesion to the walls of blood vessels. When platelets and monocytes adhere to vessel walls they induce the coagulation of blood which forms a clot. The clot can break off the vessel wall and flow to an area of constriction in the vessel where the clot cannot pass. The clot then occludes the vessel so blood does not flow leading to death of the tissue surrounding the vessel. These types of events are called embolisms and they can lead to heart failure, death of regions in the brain as well as lung impairment.
Calorie restriction has been shown to promote longevity (functions as an anti-aging regimen) in many different organisms from the simple round worm to rats and mice. In studies on the effects of calorie restriction it has been shown that life spans can be increased by as much as 40%. The pathway to longevity, induced by calorie restriction, involves the activation of the function of a protein encoded by a gene called SIRT1.
SIRT1 or sirtuin 1 is the homolog of the yeast (S. cerevisiae) Sir2 gene (Sir refers to Silent mating type Information Regulator). SIRT1 is a member of the sirtuin family of proteins (seven members in humans; SIRT1 through SIRT7) that are characterized by a sirtuin core domain and grouped into four classes. The yeast sirtuin proteins are known to regulate life-span extension, epigenetic gene silencing and suppress recombination of ribosomal DNA (rDNA).
SIRT1 is an NAD+-dependent deacetylase that modulates the activities of proteins that are in pathways downstream of the beneficial effects of calorie restriction. SIRT1 catalyzes a reaction where hydrolysis of NAD+ is coupled to the deacetylation of acetylated lysines in target proteins. These target proteins include histones, transcription factors, and transcription factor co-regulators. The NAD+ is hydrolyzed to nicotinamide (which is a strong inhibitor of SIRT1 activity) and O-acetyl-ADP ribose.
The role of resveratrol in the function of SIRT1 in calorie restriction-induced longevity was originally though to be due to direct activation of SIRT1 by resveratrol. However, subsequent research demonstrated that resveratrol activates SIRT1 indirectly through AMPK-mediated elevation in the cellular levels of NAD+.
The effects of resveratrol have been shown to increase mitochondrial content, ameliorate insulin resistance and prolong survival in laboratory mice fed a high-fat diet. Recent studies on the action of SIRT1 agonists have demonstrated that compounds that activate SIRT1, but that are structurally unrelated to resveratrol, also improve insulin sensitivity in adipose tissue, liver and skeletal muscle resulting in lower plasma glucose. The actions of these compounds in laboratory studies indicate the potential efficacy of a therapeutic approach to type 2 diabetes that includes activators of SIRT1 activity.
Berberine
Berberine [chemical name: 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a)quinolizinium] is an ammonium salt compound of the protoberberine group of benzylisoquinoline alkaloids.
Berberine is found in numerous plants of the Berberis genus of evergreen shrubs that are more commonly called barberry. The most common member of the genus is Berberis vulgaris but there are numerous popularly cultivated members such as Berberis thunbergii, Berberis candidula, and Berberis canadensis (American barberry). In addition to being found in plants of the Berberis family, berberine is present in plants of the Coptis genus (such as Coptis chinensis: Chinese goldthread), Oregon grape (Mahonia aquifolium), goldenseal (Hydrastis canadensis), and yellowroot (Xanthorhiza simplicissma). Coptis teeta has been used in China and in eastern India as a medicinal herb for treating malarial fever. Numerous parts of the Berberis plant have been used for a variety of medicinal purposes including the roots, the bark, and the fruit.
Berberine has been used in China for decades as a treatment for diarrhea. More recently the use of berberine as an herbal therapy for hyperlipidemia and type 2 diabetes has been documented. The clinical efficacy of berberine for lowering blood lipids and serum glucose has been documented over the past 10-15 years.
Numerous studies have been carried out that show a good correlation between berberine and both SIRT1 and AMPK activation. However, the only significant data demonstrating that berberine functions in the capacity as an anti-aging compound has been generated in cells in culture. In these types of experiments berberine has been shown to result in enhanced AMPK activity and increased expression of SIRT1. Increased levels of AMPK and SIRT1 are associated with metabolic pathways that promote cell survival and are thus longevity (anti-aging) inducers. The ability of berberine to activate AMPK and SIRT1 has been shown to be greater than that for resveratrol. The activation of AMPK by berberine also has the potential to augment the antimicrobial function of berberine as demonstrated in macrophages in culture.
The biggest limitation to the use of berberine supplements is the low bioavailability of the compound. Intestinal absorption of berberine is very low such that the vast majority of that consumed is eliminated, thus limiting its oral bioavailability. In addition, any berberine that is absorbed into intestinal cells is rapidly pumped back out into the lumen of the intestines via the action of a membrane transporter of the P-glycoprotein family. P-glycoprotein is also known as multidrug resistance protein 1 (MDR1). Finally, any berberine delivered to the blood from the intestines is rapidly taken up by the liver where the same membrane transporter, P-glycoprotein, pumps the berberine into the bile circulation where it is returned to the intestines for excretion.
Due to the fact that berberine is an inhibitor (weakly) of several cytochrome P450 (CYP) enzymes, specifically the CYP2D6 and CYP3A4 enzymes, there is the potential for untoward effects of berberine on prescription drugs.
Garcinia and Hydroxycitric Acid (HCA)
Garcinia gummi-gutta is a subtropical species of Garcinia native to Indonesia. The fruit rind of Garcinia gummi-gutta is most commonly known as Garcinia cambogia. Other common names include gambooge, brindleberry, brindall berry, Malabar tamarind, assam fruit, vadakkan puli and kudam puli.
Garcinia has been used for centuries in Asian countries for culinary purposes as a condiment and flavoring agent in place of tamarind or lemon, and to make meals more filling. Garcinia, or more specifically Garcinia cambogia, Garcinia atroviridis, and Garcinia indica have been found to contain large amounts of hydroxycitric acid (HCA). There are multiple chemical forms of HCA with the (-)-hydroxycitric acid form being the one found in Garcinia extracts. Another plant species with high concentrations of HCA is Hibiscus subdariffa.
Garcinia has been widely used as an anti-obesity herbal supplement for many decades. HCA has been shown to be able to inhibit the synthesis of fatty acids (a process referred to as lipogenesis) from carbohydrate (predominantly glucose) and certain amino acids. The interference in carbohydrate conversion to fat by HCA induces the body to metabolize the excess carbohydrates as well as promoting glucose storage in glycogen, both of which in turn may play a part in suppressing the appetite. HCA has also been shown to suppress appetite by increasing the release of serotonin which is a neurotransmitter that functions, in part, in the modulation of feeding behavior and appetite control.
The various chemical forms of HCA have been shown to exhibit multiple effects on metabolic processes with the consequences being the potential for weight loss and improved glucose tolerance, especially in overweight and obese individuals with type 2 diabetes.
The primary target of HCA is the enzyme called ATP citrate lyase, ACL. ACL hydrolyzes citric acid into acetyl-CoA and oxaloacetic acid. Acetyl-CoA is the required precursor for fatty acid synthesis, which occurs in all cells but at the highest levels in the liver and in adipose tissue. The primary source of the citrate for this reaction is acetyl-CoA itself derived from the metabolism of carbohydrates and also from certain amino acids. Thus, excess carbon consumption in the form of carbohydrates (sugars) or proteins will lead to fat storage and weight gain. Chemical forms of HCA have also been shown to inhibit certain digestive enzymes which results in a reduction in the ability to digest and absorb carbohydrates. This latter effect can, therefore, contribute to a reduction in blood glucose which is of great benefit in type 2 diabetes.
However, despite many research publications addressing the role of HCA as an anti-obesity supplement, which have been carried out in laboratory animals, its potential contribution as a weight loss agent in humans has been controversial. In addition, most human studies carried out so far have concluded that the safety and efficacy of HCA is questionable.
Recent meta-analysis comparing the data from multiple trials have suggested that consumption of Garcinia cambogia extracts or HCA supplementation produced only minimal short-term weight-loss, and the clinical relevance remains yet to definitively established. Most randomized double-blind placebo-controlled trials of the efficacy of Garcinia in weight loss have produced, at best, equivocal data. The largest and most rigorous randomized double-blind placebo-controlled trials to date found no significant difference in weight-loss when comparing participants who consumed HCA supplements and those who received placebo supplementation.
Of particular concern to the use of so-called “pure” Garcinia cambogia or HCA supplements is that they are sold with no verification of purity. In addition, there are various HCA salts that one can obtain in dietary supplements, including calcium, magnesium, potassium, as well as mixtures of these. When these different HCA salts are tested for efficacy in experimental animals they exhibit different properties with some, but not all, improving glucose tolerance. Several cases of liver failure have been reported in the medical literature in patients who have consumed Garcinia cambogia extracts or HCA supplements, and in one case it resulted in the need for liver transplantation for survival. Even so there have been reports that have demonstrated that HCA does not exhibit liver toxicity, although these reports involve studies in laboratory animals.
Pomegranate Phytochemicals
The pomegranate tree, Punica granatum, and especially its fruit, has a vast history of uses for the treatment of medical and health related issues. For the purposes of this discussion the pomegranate is the fruit of the Punica granatum tree which is a long-living tree cultivated throughout the Mediterranean region, as far north as the Himalayas, in Southeast Asia, and in California and Arizona in the United States.
Medically beneficial compounds can be derived from the seed, juice, peel, leaf, flower, bark, and roots of the pomegranate. Each of these anatomical compartments of the plant has interesting pharmacologic activity. For example the juice and peels possess potent antioxidant properties, while juice, peel and oil are all weakly estrogenic and have potential use for the treatment of the symptoms of menopause. The use of juice, peel and oil have also been shown to possess anticancer activities, including interference with tumor cell proliferation, cell cycle progression, tumor cell invasion and angiogenesis. These latter activities may be associated with plant based anti-inflammatory effects that are due to the potent antioxidant compounds present in the plant.
The broad scope and power of the pomegranate has been expanded recently with the discovery that the rind of the pomegranate contains antimicrobial activity that may be effective in the treatment of common hospital bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA).
The fruit of the pomegranate contains hundreds of phytochemicals, however, the antioxidant property of the fruit is thought to be due primarily to the action of ellagic acid (the main polyphenol in pomegranate) derived from ellagitannins. When pomegranates are consumed the ellagitannins are hydrolyzed, releasing ellagic acid, which is then converted to 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one derivatives (called urolithin A and urolithin B) by gut microflora.
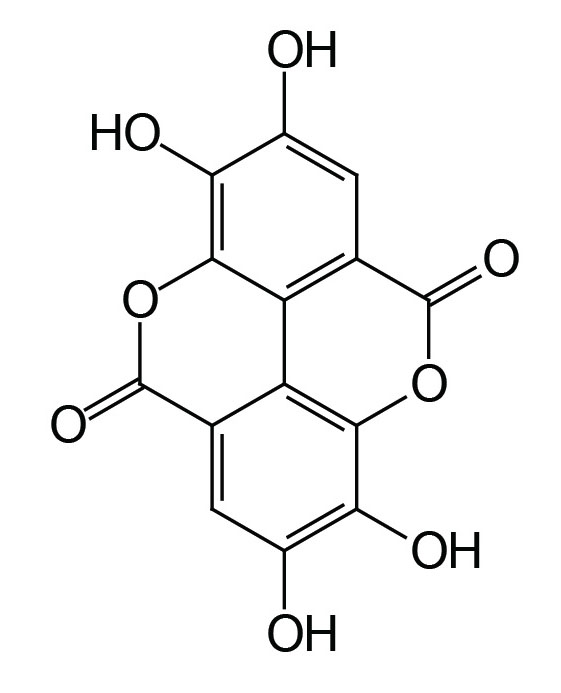
Pomegranates also contain hydrolyzable tannins in the form of punicalagins and punicalin as well as tannin-based complex oligomers that account for much of the antioxidant activity in juice.
The peel and rind of the pomegranate contains phenolic punicalagins, gallic acid and other fatty acids, catechin, epigallocatechin gallate (EGCG), quercetin, rutin, and other flavonols, flavones, flavonones, and anthocyanidins.
Pomegranate juice contains anthocyanins, glucose, ascorbic acid, ellagic acid, gallic acid, caffeic acid, catechin, EGCG, quercetin, rutin, numerous minerals, particularly iron, and amino acids.
Pomegranate seed oil is nearly 75% punicic acid (also called trichosanic acid) with small amounts of ellagic acid, sterols, and other fatty acids. Punicic acid is an 18 carbon polyunsaturated fatty acid (PUFA) that is classified as a isomer of conjugated linolenic acid. Punicic acid contains three carbon-carbon double bonds, two that exist in the cis orientation (cis9 and cis13) and one in the trans orientation (trans11). The chemical nomenclature for punicic acid is (9Z,11E,13Z)-octadeca-9,11,13-trienoic acid or more simply as (E,Z,E)-octadeca-9,11,13-trienoic acid. Once consumed punicic acid is predominantly metabolized to 9c, 11t-conjugated linoleic acid (CLA; 9c 11t-octadecadienoic acid) within the plasma as well as in other organs.
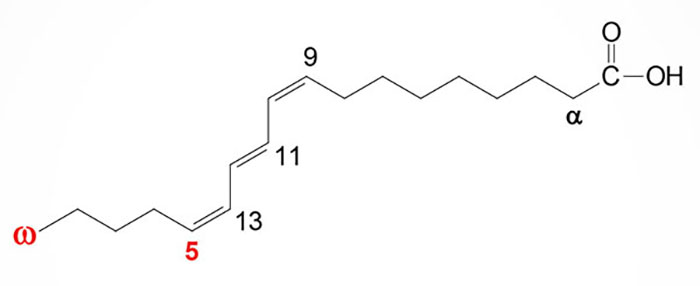
Because punicic acid is a PUFA it is often erroneously classified using the omega fatty acid nomenclature and as such is often identified as an omega-5 PUFA. However, the correct use of the omega PUFA nomenclature can only be applied to PUFA whose carbon-carbon double bonds are all in the cis orientation. Despite the confusion regarding its naming, punicic acid has been shown to have potent anti-oxidant activities and to prevent obesity in laboratory animals.
Punicic acid has been shown to exert numerous beneficial effects with the use of cell culture assays as well as in whole animal models of disease. Punicic acid administration results in a reduction in the adipose tissue content with a concomitant increase in insulin sensitivity and improvement in glucose tolerance. Punicic acid also exerts anti-inflammatory effects via inhibition of cyclooxygenase (COX) and lipoxygenase (LOX) activity.
Numerous studies on the antioxidant, anticarcinogenic, and anti-inflammatory properties of pomegranate constituents have been published, focusing on treatment and prevention of cancer, cardiovascular disease, diabetes, dental conditions, erectile dysfunction, bacterial infections and antibiotic resistance, and ultraviolet radiation-induced skin damage. Other potential applications include infant brain ischemia, male infertility, Alzheimer disease, arthritis, and obesity.
The health benefits associated with pomegranate juice have led to the development of pomegranate extracts as botanical dietary supplements. The content of ellagic acid has been used to standardize most pomegranate extract dietary supplements that are currently on the market. However, supplements can be adulterated with ellagic acid from less expensive plant sources which undermines this method of standardization.
A recent comparison was made of the phytochemical contents and antioxidant activities of 27 different commercially available pomegranate extract supplements beyond their content of ellagic acid. These supplements included capsules, tablets, and soft gels. Total phenolics were measured using both gallic acid equivalent (GAE) and ellagic acid equivalent (EAE) assays. Punicalagins, punicalin, and ellagic acid contents were determined and antioxidant capacity was measured using the Trolox equivalent antioxidant capacity (TEAC) assay. Only 5 of the tested supplements had the typical pomegranate tannin profile, 17 had ellagic acid as the predominant chemical with minor or no detectable pomegranate tannins, and 5 had no detectable tannins or ellagic acid. These results show that standardization of pomegranate extract supplements based on their ellagic acid content does not guarantee that the supplement is indeed authentic pomegranate extract. This testing demonstrates that more research is needed to assess the health impact of substituting ellagic acid for the complex mix of phytochemicals in a pomegranate extract dietary supplement.
Pomegranate extracts have been used as anticancer agents and they contain a large number of potentially bioactive substances. Punicic acid is an omega-5 long chain polyunsaturated fatty acid found in pomegranate seed oil. A number of long chain fatty acids have been reported to have cancer preventive actions. The ability of punicic acid to affect the growth of both an estrogen-insensitive breast cancer cell line (MDA-MB-231) and an estrogen-sensitive cell line (MDA-ERalpha7) has been examined. Treatment of these cells lines with 40 micromolar (μM) punicic acid inhibited proliferation up to 96% compared to untreated cells. In addition, punicic acid induced apoptosis in both cell lines and disrupted the mitochondrial membrane potential. To ascertain whether or not lipid oxidation was required for the function of punicic acid, the antioxidant tocotrienol was added to the assays. This addition resulted in a reversal of the effects of punicic acid on proliferation inhibition, apoptosis, and disruption of the mitochondrial membrane potential. The results of these studies suggest that punicic acid has breast cancer inhibitor properties that are dependent on lipid peroxidation.
Estrogen stimulates the proliferation of breast cancer cells and the growth of estrogen-responsive tumors. The aromatase enzyme, which converts androgen to estrogen, plays a key role in breast carcinogenesis. Pomegranate-derived ellagitannins have been investigated for their anti-aromatase activity and inhibition of testosterone-induced breast cancer cell proliferation. A panel of 10 ellagitannin-derived compounds including ellagic acid, gallagic acid, and urolithins A and B (and their acetylated, methylated, and sulfated analogues) were examined for their ability to inhibit aromatase activity and testosterone-induced breast cancer cell proliferation. Using a microsomal aromatase assay 6 of the ellagitannin-derived compounds exhibited anti-aromatase activity. Urolithin B was shown to be the most effective at inhibiting aromatase activity in a live cell assay. Proliferation assays also determined that urolithin B significantly inhibited testosterone-induced breast cancer cell proliferation. The rest of the tested compounds also exhibited antiproliferative activity, but to a lesser degree than urolithin B. Results from studies such as these suggest that pomegranate ellagitannin-derived compounds have potential for the prevention of estrogen-responsive breast cancers.
Recent data suggest that pomegranate-derived ellagitannins may have beneficial effects against colon cancer. Although similar compounds are found in other fruits and nuts, such as strawberries, the levels of ellagitannins are highest in pomegranates. As indicated above ellagitannins are hydrolyzed in the gut releasing ellagic acid which is then converted by gut bacteria to the urolithins (there are two primary urolithins termed urolithin A and urolithin B). The urolithins may persist in the colon through the action of the enterohepatic circulation. Currently little is known about the mechanisms of action of either the native ellagitannins or their metabolites on colon carcinogenesis.
Components of Wnt signaling pathways are known to play a pivotal role in human colon carcinogenesis, and inappropriate activation of the signaling cascade is observed in 90% of colorectal cancers. In a recent study the effects of urolithin A, ellagic acid, and ellagitannin-rich fruit extracts on Wnt signaling in a human colon cancer cell line (293T) were examined. The ellagitannin extracts, ellagic acid, and urolithin A each inhibited Wnt signaling, suggesting that ellagitannin-rich foods, such as pomegranates, have potential against colon carcinogenesis and that urolithins are relevant bioactive constituents in the colon.
Pomegranates and pomegranate extracts are also likely to have beneficial properties related to cardiovascular health. Several studies have shown that polyphenols reduce cardiovascular insults in high-risk patients; in particular, the inhibition of platelet function may be responsible for part of this benefit. Studies have been undertaken to examine the antiplatelet effects of pomegranate products containing primarily hydrolyzed tannins such as ellagitannins.
Analysis of the effects of either pomegranate juice or the polyphenol-rich extract from pomegranate fruit on platelet aggregation, calcium mobilization, thromboxane A2 (TXA2) production, and hydrogen peroxide formation, induced by collagen and arachidonic acid indicated a reduction in all platelet responses studied. These results indicate that the cardiovascular health benefits of pomegranate may in part be related to the ability of polyphenols to inhibit platelet function. In fact, pomegranate juice and pomegranate extracts have similar effects at concentrations expected following normal consumption.
Extracts from the peel of the pomegranate have demonstrated antifungal activity as well. Fractionation of crude hydroalcoholic extracts prepared from the fruit peel yields a compound (identified as punicalagin) with strong antifungal activity against Candida albicans. Of clinical significance is that the combination of punicalagin and fluconazole (Diflucan® used to treat fungal infections, including yeast infections of the vagina, mouth, throat, esophagus, abdomen, lungs, blood, and other organs and also used to treat meningitis) showed a synergistic interaction. Although these activities of punicalagin were demonstrated on the fungus in vitro, the concentrations used are achievable in vivo as well. The combination of punicalagin with fluconazole thus, represents an potentially useful treatment for the management of candidiasis.
Ergothioneine: Anti-Aging Nutrient
Ergothioneine is a naturally occurring betaine amino acid derivative of histidine. The name is derived from the fact that it was originally isolated from the ergot fungus Claviceps purpurea that normally grows on rye and other cereal grasses. Ergothioneine naturally exists in two tautomeric forms, thione and thiol, as shown in the Figure.
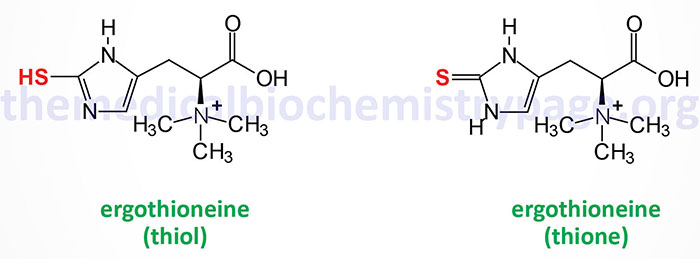
Aside from ergot fungi the richest sources of ergothioneine are mushrooms and fermented foods with the amount in in fermented foods being dependent on the species of bacteria utilized for the fermentation process. With respect to mushrooms the shiitake, oyster, king oyster, and maitake varieties contain the highest amounts of ergothioneine.
When consumed in the diet, ergothioneine is rapidly absorbed and distributed to numerous tissues. Cellular uptake of ergothioneine has been shown to occur as a result of transporters of the SLC22A family, specifically the transporters encoded by the SLC22A4 and SLC22A15 genes. The SLC22A4 encoded transporter is more commonly identified as OCTN1 (multispecific organic cation transporter 1) and, in addition to ergothioneine, it transports carnitine and acetylcholine. SLC22A4 is present in the apical membrane of intestinal enterocytes and renal tubular epithelial cells where it functions in the uptake of ergothioneine from the diet and reabsorption from the glomerular filtrate, respectively. In humans the highest levels of expression of SLC22A4 are in erythroid progenitor cells, neutrophils, and monocytes.
Ergothioneine functions as an antioxidant and has been shown to improve both health-span and life-span. The antioxidant capacity of ergothioneine is exerted at the level of hydroxyl radicals, singlet oxygen species, and reactive nitrogen species. The reactivation of ergothioneine following its oxidation by any of these reactive molecules occurs directly via ascorbate as well as enzymatically by glutathione reductase (in the presence of glutathione) or thioredoxin reductase.
Recent evidence has demonstrated that ergothioneine can exert positive effects on health-span and life-span by functioning as a substrate for enzymatic hydrogen sulfide (H2S) production by cystathionine γ-lyase (cystathionase) and by cystathione β-synthase (CBS). Hydrogen sulfide is classified as a gasotransmitter because of its ability to induce smooth muscle relaxation and vasodilation. Hydrogen sulfide also induces protein persulfidation, a post-translational modification of the R-group of cysteine residues.
The significance of ergothioneine function, leading to health-span improvement, was demonstrated by experiments showing that cystathionine γ-lyase was required for the persulfidation of the cytosolic form of glycerol-3-phosphate dehydrogenase (cGAPDH; encoded by the GPD1 gene). The GPD1 encoded enzyme is often designated cGPDH. The cystathionine γ-lyase-mediated persulfidation of cGAPDH directly contributes to the increased production of NAD+ via the reduction of glyceraldehyde-3-phosphate to glycerol-3-phosphate and the oxidation of NADH to NAD+. Levels of NAD+ are known to fall as humans age and experiments have demonstrated that methods of increasing NAD+ levels contribute to enhanced longevity in mammals and invertebrates.
Exercise, which enhances the activity of the transcriptional co-activator, PGC-1α, results in increased expression of the SLC22A4 gene in cardiac and skeletal muscle cells resulting in increased ergothioneine uptake. Ergothioneine binds to one of the cysteine catabolic enzymes, mercaptopyruvate sulfurtransferase (encoded by the MPST gene). The MPST encoded enzyme is also known as cysteine aminotransferase/mercaptopyruvate sulfurtransferase. MPST converts mitochondrial 3-mercaptopyruvate (3-MP) to pyruvate and hydrogen sulfide, H2S. Enhanced H2S production in the mitochondria contributes to increased electron flow and mitochondrial biogenesis, both of which promote cellular health and longevity.
