Last Updated: October 31, 2025
Introduction to Huntington Disease
Huntington disease is inherited as an autosomal dominant disorder characterized by slowly progressive neurodegeneration associated with choreic movements (abnormal involuntary movements) and dementia. The disease is named after George Huntington who provided the classical account of the disease in 1872. The pathology of HD reveals neurodegeneration in the corpus striatum and shrinkage of the brain.
In addition, HD exhibits a genetic phenomenon termed “anticipation” which means that the symptoms of the disease appear earlier and are more severe in subsequent generations. This phenomenon is explained by meiotic instability which increases the CAG repeat number and is greater in spermatogenesis than in oogenesis. Therefore, anticipation in mainly observed when the mutation is inherited through the paternal line.
Molecular Biology of Huntington Disease
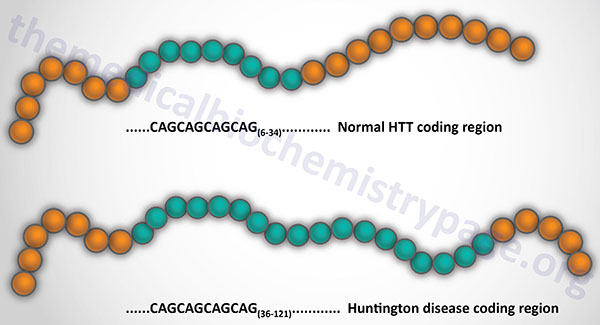
HD is caused by expansion of a CAG trinucleotide repeat in the first exon of the huntingtin (HTT) gene. The HTT gene is located on chromosome 4p16.3, spans over 200 kb, and is composed of 67 exons that generate two alternatively polyadenylated mRNAs that are present at different levels in different fetal and adult tissues. These mRNAs encode proteins (in the normal non-triplet expanded state) of 3142 amino acids and 3144 amino acids. Due to individual differences in the number of Gln (Q) residues in the N-terminus of the huntingtin protein the total number of amino acids in unaffected individuals ranges between 3144 and 3172.
The HTT gene is ubiquitously expressed and generates two mRNAs that differ in the length of their 3′ untranslated regions. The CAG repeat resides 17 codons downstream of the initiator AUG codon. The range of CAG repeat size in normal individuals is 6–34 and in affected individuals it is 36–121. The longer the length of the CAG repeat the earlier is the onset of symptoms. However, there is a wide degree of variation in age of onset for any given length of CAG repeat. Thus, the CAG repeat number itself is not predictive for age of onset. The variation in age of onset is related to the effects of other modifying genes, the environment, and the length of the CAG repeat.
Huntingtin Functions
The huntingtin protein is unrelated to any other protein. The polyglutamine tract begins at codon 18 and is followed by a stretch of 29 consecutive proline residues. Wild-type huntingtin protein is found primarily in the cytoplasm with a small percentage being intranuclear. The protein is also found associated with the plasma membrane, the endoplasmic reticulum (ER), the Golgi apparatus, endocytic vesicles, the mitochondria, and microtubules.
In addition to the polyglutamine and polyproline motifs, huntingtin contains 28–36 repeats, termed HEAT motifs, dispersed throughout the length of the protein (HEAT = huntingtin, elongation factor 3, the PR65/A subunit of protein phosphatase 2A and the lipid kinase Tor). The HEAT motif is approximately 50 amino acids in length and is composed of two anti-parallel α-helices that form a hairpin structure. HEAT motifs are involved in protein-protein interactions and serve roles in intracellular transport, chromosome segregation, and microtubule dynamics.
There is no clear nuclear localization signal in huntingtin (although a stretch of basic amino acids from position 1182-1190 can confer nuclear localization when fused to bacterial β-galactosidase) but there is a nuclear export signal in the C-terminus of the protein.
Huntingtin is also fatty acid modified at cysteine 214 (C214) with palmitic acid. The acylation (S-palmitoylation) is catalyzed by Hip14 (huntingtin interacting protein 14). This modification regulates huntingtin function and trafficking. The significance of the palmitoylation is demonstrated by the fact that huntingtin protein with an expanded polyglutamine tract is a much poorer substrate for Hip14 than is wild-type protein. Although numerous studies have been aimed at elucidating the exact function of huntingtin, its cellular roles are still poorly defined.
In spite of the lack of a clearly defined role(s) for huntingtin, much has been learned about possible involvement of the protein in numerous processes. Huntingtin is essential for early embryonic development as evidenced by lethality in embryonic mice lacking functional protein. The protein is known to be important in adult neurons and testes for cellular viability. If the level of the protein is reduced it leads to developmental defects and disruptions in iron homeostasis.
Huntingtin also has a role in regulation of transcription through its interaction with numerous transcription factors and other proteins involved in the regulation of mRNA production.
Huntingtin is highly expressed in the presynaptic area of neurons where it interacts with multiple proteins involved in synaptic vesicle exocytosis and recycling.
An additional role for huntingtin in vesicle trafficking is speculated given its localization to endocytic/endosomal vesicles and from its interactions with a number of endocytic/trafficking proteins such as clathrin, dynamin, α-adaptin, Hip1, Hip14 and Hap1 (huntingtin associated protein 1).
Huntington Disease Modifiers
Whereas the length of the polyglutamine tract in the huntingtin protein can clearly be correlated with disease, only about 60% of the variation in the age of onset can be accounted for by the polyglutamine expansion. Genome wide association studies (GWAS) have identified several genetic loci associated with the age of onset of Huntington disease but that have no effect in the absence of the expansion of the polyglutamine tract in the huntingtin protein. Many of these modifier loci encode genes involved DNA repair, in particular DNA mismatch repair (MMR), and contribute to the somatic instability of the CAG repeat in the HTT gene.
These modifier genes include MLH1, MSH3, PMS1, PMS2, and FAN1. MLH1 and MSH3 are the human homologs of the bacterial MutL family and MutS family of proteins, respectively. MLH1 stands for MutL homolog 1 and MSH3 stands for MutS homolog 3. The PMS1 and PMS2 refers to Post Meiotic Segregation increased 1 and 2, respectively. In humans, mismatch recognition is mediated by one of two heterodimeric protein complexes. One of these complexes is composed of MLH1 and PMS2. The FAN1 gene encodes a nuclease that is found in a complex with several proteins of the Fanconi anemia complementation group of proteins. Fanconi anemia represents a family of inherited cancer syndromes that includes at least 21 complementation groups that includes FANCD2 and FANCI whose encoded proteins complex with the FAN1 encoded protein.
Clinical Features of Huntington Disease
The classical symptoms of Huntington disease consist of progressive dementia, evolving involuntary movements and psychiatric disturbances that include personality changes and mood disorder. Choreiform movements are the most prominent physical abnormality in HD and as such the disease was early on referred to as Huntington chorea. Choreiform movements are characterized by repetitive, brief, irregular, somewhat rapid involuntary movements typically in the face, mouth, trunk, and limbs. The earliest indications of HD is the appearance of spasmodic twitching of the extremities, generally beginning with the fingers. Additional early physical manifestations of HD are clumsiness, hyperreflexia, and eye movement disturbances. Positron-emission tomography (PET scanning) is used to demonstrate a loss of uptake of glucose in the caudate nuclei and is a valuable indication of affectation in the pre-symptomatic period in HD patients.
Individuals with Huntington disease usually begin to manifest symptoms between the ages of 35 and 40 years. The duration of the disease is typically around 15–20 years from the onset of motor dysfunction. The length of the course of the disease is , independent of the number of CAG repeats. The typical clinical course for Huntington disease can be divided into three stages. The first stage is the pre-symptomatic stage where there are no detectable clinical abnormalities. The second phase is termed the prodromal phase. In this second phase affected individuals begin to experience subtle changes in motor skills, cognitive ability, and behavior. These changes are subtle and often are only recognizable by close family members. The third and final stage of the disease is referred to as the manifest stage. This stage when clinical diagnosis of Huntington disease is made.
The neuropathology of Huntington disease is due to effects in the basal ganglia, principally within the medium spiny neurons (MSN) of the striatum. MSN are responsible for motor control and reward pathways. These neurons are inhibitory neurons that utilize the neurotransmitter, GABA. The basal ganglia are involved in numerous functions including voluntary and involuntary movement, cognition, emotion, procedural learning, and habit formation. Due to their role in movement and cognition, defects in the basal ganglia are associated with many types of movement disorder such as parkinsonism and dyskinesias, as well as alterations in mood and lead to obsessive compulsive disorders.
Juvenile Huntington Disease
There is a rare form of early onset Huntington disease termed juvenile Huntington disease. This form of the disease, representing around 5% of all cases of Huntington disease, manifests in individuals younger than 20 years. In juvenile Huntington disease the number of CAG repeats is greater than 60 and in some cases as many as 80 repeats have been identified.
Juvenile Huntington disease is associated with many symptoms that are indicative of the adult-onset disease with the exception that choreic movements are rare. The predominant symptoms in juvenile Huntington disease are bradykinesia (slowness of movement), akinesia (inability to perform a clinically perceivable movement), and rigidity. Progressive cognitive decline is also common in juvenile Huntington disease patients resulting in intellectual impairment and learning disability.
Treatments for Huntington Disease
Currently there is no cure for HD and all afflicted individuals will succumb to the disease. However, there are several avenues of treating Huntington disease, many of which focus on the symptoms. Drugs that target the dopaminergic pathway in the brain to treat the movement dysfunction in Huntington disease have shown positive results. These drugs include deutetrabenazine and etrabenazine that inhibit the vesicular monoamine transporter (VMAT2) and thereby decrease the bioavailability of dopamine in synapses resulting in reduced dopamine signaling.
Promising results have been demonstrated with the use of RNAi-mediated technologies. These technologies involve the injection of vectors expressing huntingtin mRNA targeting small interfering RNAs (siRNA) that when expressed in neurons result in degradation of the mRNA encoding the abnormal huntingtin protein. Recent results have shown up to 75% reduction in the progression of Huntington disease symptoms during the course of a three year trial utilizing an adeno-associated virus (AAV) vector expressing a miRNA targeting the huntingtin mRNA..
.