Last Updated: October 28, 2025
Gangliosides
Gangliosides are members of the glycosphingolipid family of glycolipids. Gangliosides are glycosphingolipids that are defined by the presence of N-acetylneuraminic acid (NANA; also known as sialic acid) in varying amounts. The specific names for gangliosides are a key to their structure. The first letter on the nomenclature, G, refers to ganglioside. The following letters, A, M, D, T, Q, and P, indicate that the molecule contains none (absent or asialo), mono-, di-, tri, quatra(tetra), or poly(more than 4)-sialic acid residues. The numerical designations, 1, 2 and 3, refer to the carbohydrate sequence that is attached to ceramide, see the Sphingolipid Metabolism and the Ceramides page for details.
Introduction to GM1 Gangliosidosis
GM1 gangliosidosis belongs to a family of disorders identified as lysosomal storage diseases. This disorder is an autosomal recessive disease characterized by the lysosomal accumulation of glycoconjugates with terminal β-galactosyl residues. These glycoconjugates accumulate in the lysosomes as a consequence of defects in the lysosomal hydrolase, β-galactosidase-1. The major groups of compounds that are seen accumulating in patients with defects in β-galactosidase-1 are GM1 gangliosides, the asialo derivatives of GM1 gangliosides (GA1), keratan sulfates, and glycoprotein-derived oligosaccharides.
Molecular Biology of GM1 Gangliosidosis
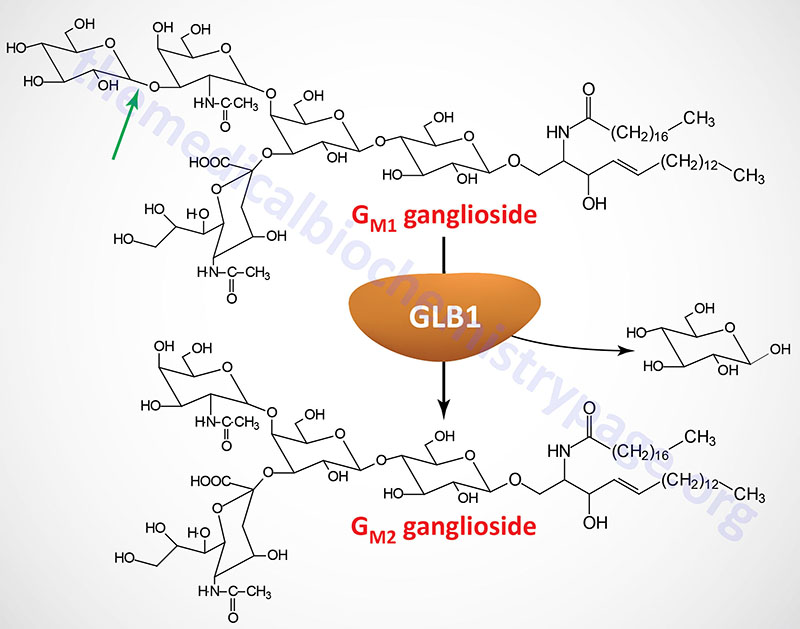
The β-galactosidase 1 enzyme is encoded by the GLB1 gene. The GLB1 gene is located on chromosome 3p22.3 and is composed of 19 exons that generate five alternatively spliced mRNAs, each of which encode a distinct protein isoform.
The GLB1 gene belongs to the glycoside hydrolase 35 family which is a family within the large glycoside hydrolase superfamily (14 major families). The major GLB1 encoded protein is a 677 amino acid precursor protein that is processed to a 654 amino acid glycoprotein. Functional β-galactosidase 1 functions in a complex with protective protein/cathepsin A (PPCA) and saposin B. See the Sphingolipid Metabolism and the Ceramides page or the Gaucher disease page for a description of the activities of the saposins. The activity of PPCA is to stabilize β-galactosidase.
The major substrates for β-galactosidase are GM1 gangliosides, GA1 (an asiolo GM1 ganglioside), lactosylceramides, asialofetuin, keratan sulfates, and galactose-containing oligosaccharides. In addition to the action of β-galactosidase on terminal β-linked galactose, another enzyme, identified as galactosylceramidase (galactocerebrosidase), exhibits a similar activity but with substrate preference towards galactosylceramides, galactosylsphingosines, lactosylceramides, and monogalactosyl diglycerides. Deficiencies in galactosylceramidase result in Krabbe disease (globoid cell leukodystrophy, GLD).
Clinical Features of GM1 Gangliosidosis
GM1 gangliosidosis is a disease encompassing the nervous system and numerous visceral organs. In typical cases the disease is diagnosed in early infancy. However, the clinical manifestations of GM1 gangliosidosis are heterogeneous and thus the disease is divided into 3 sub-types:
Infantile (type 1)
Clinical signs of the disease appear early in infancy after a period of normal development. However, cases have been described where neurologic involvement was evident immediately after birth. In most cases infants exhibit arrest or delay in developmental milestones beginning between 3 and 6 months of age. Their ability to feed is impaired and they exhibit poor suckling behavior. Signs of severe psychomotor impairment become obvious with functional decline in nervous system function in the next few months.
Affected infants will have corneal clouding and the presence of macular pallor (the cherry-red spot) in the eye typical of that seen in Tay-Sachs disease, another lysosomal storage disease. Infants will show an exaggerated startle response to sounds. At the outset of the disease there is generalized hypotonia but as the disease progresses spasticity and frequent seizures occur.
Typical dysmorphic and skeletal dysplasia is evident and progressive. Infants have coarse thick hair, a depressed nasal bridge, gingival hypertrophy, large low-set ears and hair on the forehead. As the disease progresses the afflicted individual will become vegetative with death occurring within a few years of the onset of disease.
Late infantile/juvenile (type 2)
Progression of type 2 disease is highly heterogeneous with most of the symptoms seen in type 1 disease absent or greatly reduced in severity. Symptoms are often observed around 3 months of age with progressive deterioration occurring between 12 and 18 months of age but visceromegaly, dysmorphisms and cherry-red spots are usually not present. The course of type 2 disease occurs over a period of 1 to 5 years.
Adult chronic (type 3)
In the adult form of the disease symptoms are not generally evident until at least 3 years of age but often not until the second decade. This form of the disease is much slower in progression as well occurring over a 10 to 30 year period. Dystonia is the major neurological finding in the adult form of the disease. Disturbances in speech and walking are usually the first signs in most adult onset patients. Some patients exhibit corneal clouding but cherry-red spots are not present.