Last Updated: August 6, 2025
Galactose Metabolism
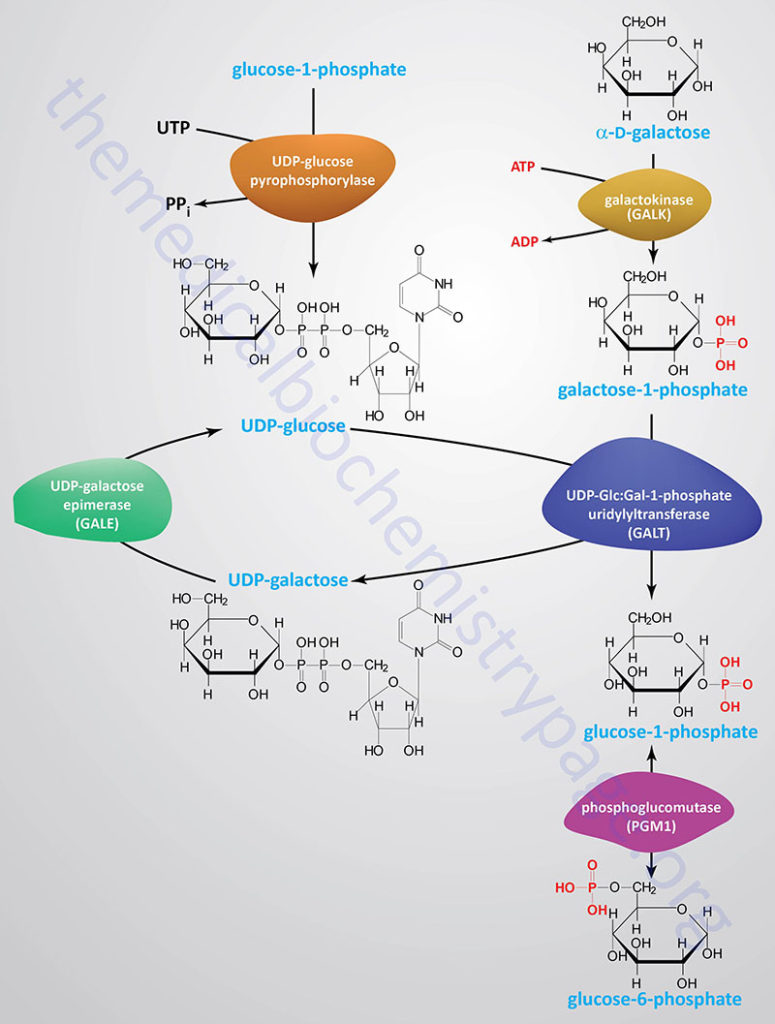
Galactose, which is metabolized from the milk sugar, lactose (a disaccharide of glucose and galactose), enters glycolysis by its conversion to glucose-1-phosphate (G1P). This occurs through a series of steps that is referred to as the Leloir pathway, named after Luis Federico Leloir who determined the overall process of galactose utilization.
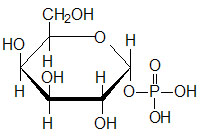
Galactose can exist in two different stereoisomeric forms; α-D-galactose and β-D-galactose. The α-form is that which is metabolized in the Leloir pathway. Conversion of the β-form of galactose to the α-form requires the enzyme galactose mutarotase encoded by the GALM gene (also known as aldose 1-epimerase).
The GALM gene is located on chromosome 2p22.1 and is composed of 9 exons that encode a 342 amino acid protein. Expression of the GALM gene is highest in the kidneys and small intestine, primarily the duopdenum.
Galactokinase
The first reaction of the Leloir pathway is the phosphorylation of α-D-galactose by galactokinase to yield galactose-1-phosphate. The galactokinase protein is encoded by the GALK1 gene. The GALK1 gene is located on chromosome 17q25.1, spans 7.3 kbp, and contains 8 exons that encode a 392 amino acid protein.
There is another gene identified as GALK2 that was originally thought to be a second galactokinase encoding gene but was subsequently shown to be an N-acetylgalactosamine kinase.
Galactose-1-Phosphate Uridylyltransferase
Epimerization of galactose-1-phosphate to glucose-1-phosphate (G1P) requires the transfer of UDP from UDP-glucose catalyzed by galactose-1-phosphate uridylyltransferase encoded by the GALT gene.
The GALT gene is located on chromosome 9p13.3 and is composed of 11 exons that generate two alternatively spliced mRNAs that encode two distinct isoforms of the enzyme. GALT isoform 1 is a protein of 379 amino acids and GALT isoform 2 is a protein of 270 amino acids. The GALT catalyzed reaction generates UDP-galactose and glucose-1-phosphate.
UDP-Galactose-4 Epimerase
The UDP-galactose is epimerized to UDP-glucose by UDP-galactose-4 epimerase which is encoded by the GALE gene. The UDP portion is exchanged for phosphate generating glucose-1-phosphate which then is converted to glucose-6-phosphate (G6P) by phosphoglucose mutase. The GALE encoded enzyme catalyzes two distinct but analogous epimerization reactions, the epimerization of UDP-galactose to UDP-glucose and the epimerization of UDP-N-acetylgalactosamine to UDP-N-acetylglucosamine.
The GALE gene is located on chromosome 1p36.11 and is composed of 13 exons that generate three alternatively spliced mRNAs all of which encode the same 348 amino acid protein.
Alternative Pathways of Galactose Metabolism
There are additional minor pathways of galactose metabolism in humans that do not involve all three of the enzymes of the classical Leloir pathway. Under normal conditions, each of these alternative pathways are responsible for the metabolism of only trace quantities of galactose.
Galactose can be converted to UDP-glucose by the sequential activities of GALK, UDP-glucose pyrophosphorylase 2 (UGP2), and GALE.
Galactose can also be reduced to galactitol by NADPH-dependent aldose reductase. This latter reaction becomes significant in the context of GALT and GALK1 deficiencies that result in galactosemias (also described below).
Finally, galactose can be oxidized to galactonate via an NAD+-dependent reaction, however, the enzyme in humans has yet to be characterized. The conversion of galactose to galactonate is significant only in individuals harboring mutations in the GALT gene.
Disorders of Galactose Metabolism
Classic Galactosemia: Type 1
Classic galactosemia refers to a disorder arising from profound deficiency of the enzyme galactose-1-phosphate uridylyltransferase (GALT) and is termed type 1 galactosemia. There are also classified clinical and biochemical variant forms of GALT deficient galactosemia. Classic galactosemia is inherited as an autosomal recessive disorder and almost all afflicted individuals will present with symptoms in the neonatal period if undiagnosed. Type 1 galactosemia occurs with a frequency of between 1:40,000 to 1:60,000 live births in Western countries. When the frequency of galactosemia inheritance is assessed by anyone with erythrocyte GALT activity <5% of normal and erythrocyte galactose-1-phosphate concentration >2mg/dL then the frequency reaches 1:10,000. Certain countries have higher levels of classic galactosemia inheritance than Western countries such as in Ireland where the frequency is close to 1:16,000 live births.
Classic galactosemia manifests by poor feeding behavior, a failure of neonates to thrive, bleeding problems, and E. coli-associated sepsis in untreated infants. In classic glactosemia, infants will exhibit vomiting and diarrhea rapidly following ingestion of lactose from breast milk or from lactose-containing formula. Due to the rapid progression of the symptoms, infants are sometimes erroneously termed lactose intolerant. However, the clinical distinction between classic lactose intolerance and classic galactosemia is quite profound. Newborn screening for classic galactosemia occurs in most states in the US and thus, the risks to newborns with this disease are drastically reduced. The typical prenatal test for galactosemia involves measurements of both erythrocyte GALT activity and erythrocyte levels of galactose-1-phosphate, the former being significantly reduced or absent, and the latter being elevated. Some prenatal tests only measure total blood galactose levels and in infants afflicted with a clinical or biochemical variant type 1 galactosemia, this assay may not be sufficient to detect any abnormality. In some cases of clinical or biochemical variant type 1 galactosemia a genetic test for GALT variant mutations may be necessary to confirm a diagnosis.
Clinical findings in infants with classic galactosemia who consume lactose or galactose containing meals include impaired liver function (which if left untreated leads to severe cirrhosis), hypoglycemia, hyperbilirubinemia, elevated blood galactose, hypergalactosemia, hyperchloremic metabolic acidosis, urinary galactitol excretion and hyperaminoaciduria. Unless controlled by exclusion of galactose from the diet, these galactosemias can go on to result in fatal liver failure, brain damage, and blindness. Blindness is due to the conversion of circulating galactose to the sugar alcohol galacitol, by an NADPH-dependent aldose reductase that is present in neural tissue and in the lens of the eye. At normal circulating levels of galactose this enzyme activity causes no pathological effects. However, a high concentration of galacitol in the lens causes osmotic swelling, with the resultant formation of cataracts and other negative symptoms within the eye.
The principal treatment for infants with classic galactosemia, whose erythrocyte GALT activity is <10% of normal, is elimination of lactose from the diet. This lactose elimination regimen includes breast milk and all other milk products. Even on a galactose-restricted diet, GALT-deficient individuals exhibit urinary galacitol excretion and persistently elevated erythrocyte galactose-1-phosphate levels. In addition, even with life-long restriction of dietary galactose, many patients with classic galactosemia can develop serious long-term complications including speech defects, cognitive impairment, and in female patients the potential for premature follicular atresia resulting in ovarian insufficiency and sterility. Due to these persistent clinical complications, it is recommended that individuals with classic galactosemia undergo routine testing for the accumulation of erythrocyte galactose-1-phosphate, increased urinary galactose output, developmental delay, speech problems, and the formation of cataracts.
Type 2 Galactosemia
The second form of galactosemia, termed type 2, results from deficiency of galactokinase (GALK1). This form of galactosemia is quite rare with a frequency of <1:100,000 live births. Infants with GALK deficiency, who continue to consume a milk-based diet, accumulate abnormally high levels of galactose in their blood and tissues, similar to infants with classic galactosemia. Like classic galactosemia patients GALK deficiency often presents with cataracts that will resolve upon dietary restriction of galactose. However, unlike patients with classic galactosemia, patients with GALK deficiency who keep to a galactose restricted diet experience no known long-term complications. This difference is biochemically and clinically quite significant because it provides compelling evidence that it is not the accumulation of galactose, but rather galactose-1-phosphate (Gal-1P), or possibly some metabolic derivative Gal-1P, that is the primary cause of the complications, in addition to cataracts, that are observed in classic galactosemia patients and the more rare severe form of GALE deficiency.
Type 3 Galactosemia
The third disorder of galactose metabolism, termed type 3 galactosemia, results from a deficiency of UDP-galactose-4-epimerase (GALE). Two different forms of this deficiency have been found. The more commonly occurring deficiency affects only red and white blood cells and is relatively benign. The other form of GALE deficiency is extremely rare (only 8 known cases) and is characterized by profound enzyme impairment affecting multiple tissues and manifesting with symptoms similar to those seen with GALT deficiency (classic galactosemia).