Last updated: February 18, 2026
Introduction to Disorders of Fatty Acid Oxidation
The majority of clinical problems related to fatty acid metabolism are associated with the processes of mitochondrial fatty acid β-oxidation but also include disorders in peroxisomal lipid metabolism. Disorders that result from defects in mitochondrial fatty acid β-oxidation fall into three main groups: defects/deficiencies in carnitine, carnitine palmitoyltransferase deficiencies, and acyl-CoA dehydrogenase deficiencies.
Disorders of peroxisomal fatty acid oxidation can result from defects in single peroxisomal enzymes or can be the result of defects in the biogenesis of the peroxisomes. The latter group of disorders are referred to as peroxisomal biogenesis disorders (PBD) the most severe of which are the Zellweger syndrome disorders.
Deficiencies in Carnitine
Deficiencies in carnitine can be either primary or secondary. Primary carnitine deficiencies are due to defects in carnitine transport into cells or due to defects in synthesis from lysine. Mutations in the major carnitine transporter, encoded by the SLC22A5 gene (also known as zwitterion/cation transporter 2 (OCTN2), are the predominant causes of primary carnitine deficiency (correctly identified as carnitine deficiency, systemic primary).
The SLC22A5 gene is located on chromosome 5q31.1 and is composed of 11 exons that generate two alternatively spliced mRNAs encoding proteins of 581 amino acids (isoform a) and 557 amino acids (isoform b). The SLC22A5 gene is ubiquitously expressed with highest levels seen in the small intestine and the kidneys.
Secondary carnitine deficiencies are most commonly the result of trapping of carnitine in fatty acylcarnitines due to defects in mitochondrial fatty acid β-oxidation enzymes.
Carnitine deficiencies lead to an inability to transport long-chain fatty acids into the mitochondria for oxidation. Carnitine deficiencies can occur in newborns and particularly in pre-term infants. Carnitine deficiencies also are found in patients undergoing hemodialysis or those affected by any one of various organic acidemias/acidurias (e.g. propionic acidemia or isovaleric acidemia).
Carnitine deficiencies may manifest with systemic symptomology or may be limited to only muscles. Symptoms can range from mild occasional muscle cramping to severe weakness or even death. Treatment is by oral carnitine administration.
Carnitine Palmitoyltransferase Deficiencies
Deficiencies in CPT1 are relatively rare and affect primarily the liver and lead to reduced fatty acid oxidation and ketogenesis. The most common symptom associated with CPT1 deficiency is hypoketotic hypoglycemia associated with hepatic pathology. There is also an elevation in blood levels of carnitine. The liver involvement results in hepatomegaly and the muscle involvement results in weakness.
CPT2 deficiencies can be classified into three main forms. The adult form is the most common and affects primarily the skeletal muscles and is called the adult myopathic form. This form of the disease causes muscle pain and fatigue and myoglobinuria following prolonged exercise. This form of CPT2 deficiency manifests with symptoms that are very similar to those exhibited by patients with the glycogen storage disease, McArdle disease. However, with McArdle disease the muscle pain and cramping is associated with short bursts of aerobic exercise, such as following sprinting, as opposed to following sustained exercise as in the case of CPT2 deficiency.
The severe infantile multisystem form manifests in the first 6–24 months of life with most afflicted infants demonstrating significant involvement before 1 year. The primary symptom of this form of CPT2 deficiency is hypoketotic hypoglycemia. Symptoms will progress to severe hepatomegaly and cardiomyopathy. Often times death from CPT2 deficiency may be misdiagnosed as sudden infant death syndrome, SIDS.
The rarest form of CPT2 deficiency is referred to as the neonatal lethal form. Symptoms of this form appear within hours to four days after birth and include respiratory failure, hepatomegaly, seizures, hypoketotic hypoglycemia, and cardiomegaly. The cardiomegaly will lead to fatal arrhythmias.
Carnitine acyltransferases may also be inhibited by sulfonylurea drugs, such as tolbutamide and glyburide, that are used in the treatment of the hyperglycemia of type 2 diabetes.
Carnitine Acylcarnitine Translocase Deficiency
Mutations in the SLC25A20 gene that encodes the carnitine acylcarnitine translocase (CACT) are associated with a rare autosomal recessive disorder. Fewer than 40 cases of CACT deficiency have been described worldwide. Carnitine acylcarnitine translocase deficiency manifests in the neonatal period (within hours of birth) with breathing difficulty, irregular heart beat, and seizures. Deficiency of CACT can induce lethal neonatal episodes of coma due to hypoketotic hypoglycemia, cardiomyopathy, cardiac arrhythmia and rhabdomyolysis. Metabolic consequences of CACT deficiency are hypoketotic hypoglycemia, dicarboxylic aciduria, elevated serum levels of long-chain acylcarnitines, reduced serum carnitine, and hyperammonemia.
Deficiency in CACT is difficult to differentiate from severe cases of CPT2 deficiency solely on the basis of clinical manifestations and blood acylcarnitine profiles. Given that deficiency of CACT is extremely rare, infants manifesting with hypoketotic hypoglycemia, respiratory failure, hepatomegaly, seizures, and cardiomegaly are more likely to have a deficiency in CPT2. Molecular analysis is the only definitive mechanism to distinguish these two disorders.
The SLC25A20 gene is located on chromosome 3p21.31 and is composed of 9 exons spanning approximately 42 kb that encode a 301 amino acid protein. The SLC25A20 gene is ubiquitously expressed with highest levels seen in the small intestine and liver.
Deficiencies in Acyl-CoA Dehydrogenases
The acyl-CoA dehydrogenase deficiency disorders represent a group of inherited disorders that are associated with impairment of mitochondrial fatty acid β-oxidation. As the name implies, these disorders result from deficiencies in the family of acyl-CoA dehydrogenases that catalyze the first reaction in the mitochondrial β-oxidation of long-chain fatty acids. The enzymes affected belong to one of three categories:
- Very long-chain acyl-CoA dehydrogenase (VLCAD): The symptoms that result from deficiencies in VLCAD were originally ascribed to loss of long-chain acyl-CoA dehydrogenase (LCAD) activity. However, expression of the gene encoding LCAD (ACADL) is very low and restricted in humans and, therefore, LCAD does not contribute, to any significant extent, to long-chain fatty acid oxidation. Mitochondrial oxidation of long-chain fatty acids begins through the activity of VLCAD.
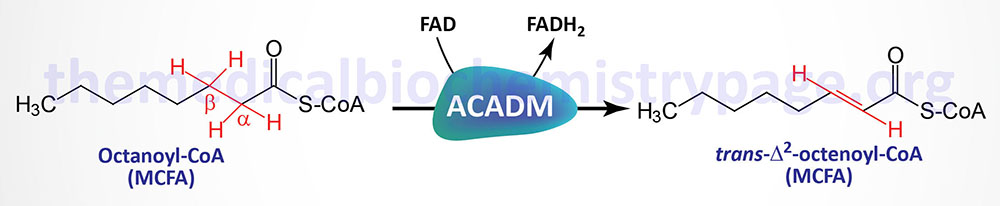
- Medium-chain acyl-CoA dehydrogenase (MCAD): MCAD deficiency (MCADD) is the most common form of acyl-CoA dehydrogenase deficiency. In the first years of life this deficiency will become apparent following a prolonged fasting period. Symptoms include vomiting, lethargy and frequently coma. Excessive urinary excretion of medium-chain dicarboxylic acids as well as their glycine and carnitine esters is diagnostic of this condition. In the case of this enzyme deficiency taking care to avoid prolonged fasting is sufficient to prevent clinical problems.
- Short-chain acyl-CoA dehydrogenase (SCAD)
Mitochondrial Trifunctional Protein (MTP) Deficiency
In addition to deficiencies in the fatty acyl-CoA dehydrogenases, that catalyze the first reaction of mitochondrial long-chain fatty acid β-oxidation, deficiencies in the activities of the mitochondrial trifunctional protein (MTP), the enzyme complex that catalyzes the last three reactions of mitochondrial long-chain fatty acid β-oxidation, have also been identified. The MTP is composed of α-subunits and β-subunits encoded by the HADHA and HADHB genes, respectively.
The HADHA gene is located on chromosome 2p23.2 and is composed of 20 exons that encode a 763 amino acid precursor protein.
The HADHB gene is also located on chromosome 2p23.3 in a head-to-head orientation with the HADHA gene. The HADHB gene is composed of 17 exons that generate three alternatively spliced mRNAs, each of which encode a distinct protein isoform.
Mutations in the HADHA gene represent one form of the MTP deficiency syndromes and complete loss of MTP activity represents the other. Because the HADHA gene encoded enzyme possesses long-chain L-3-hydroxyacyl-CoA dehydrogenase (LCHAD) activity the disorder is also commonly referred to as long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCAHDD).
Missense mutations in the HADHB gene have been identified that result in the loss of the long-chain 3-keto-acyl-CoA thiolase activity. These HADHB mutations are collectively part of the MTP deficiency spectrum. Females harboring HADHB mutations are at risk of developing a disorder termed acute fatty liver of pregnancy (AFLP) as well as the disorder referred to as hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome during pregnancy.
MTP deficiency is an autosomal recessive disorder with a frequency of 1 in 100,000. The clinical symptoms associated with MTP deficiency include hypoketotic hypoglycemia, metabolic acidosis, metabolic encephalopathy, liver dysfunction, cardiomyopathy, exercise-induced myoglobinuria, and rhabdomyolysis. MTP deficiency can sometimes be mistaken as Reye syndrome.
Total loss of MTP activity is often associated with a very high rate of early lethality. However, some patients with total MTP deficiency have been reported that exhibit a much less severe phenotype, with peripheral neuropathy being the only prominent symptom. To date, a total of 72 mutations have been identified in the HADHA gene and 67 mutations in the HADHB gene that are associated with the symptoms of MTP deficiency.
Lipid Storage Myopathies (LSM)
The lipid storage myopathies (LSM) represent a heterogeneous group of inherited disorders that, in addition to other pathologies, are all characterized by abnormal lipid accumulation in muscle fiber. The LSM are classified based upon pathological findings which result, in large part, due to the accumulation of lipid droplets (LD) in muscle but also in other tissues.
Four discernable LSM have been categorized and includes primary carnitine deficiency (PCD), multiple acyl-CoA dehydrogenase deficiency (MADD; also known as glutaric acidemia type II, GAII), and the neutral lipid storage diseases (NLSD) which includes NLSD with myopathy (NLSDM) and NLSD with ichthyosis (NLSDI).
MADD is a disorder that results from mutations in either of the two genes that encode the protein components of the heterodimeric complex identified as electron transfer flavoprotein (ETF) or the gene encoding electron transfer flavoprotein dehydrogenase (encoded by the ETFDH gene). The ETF is composed of an α-subunit encoded by the ETFA gene and a β-subunit encoded by the ETFB gene. The ETF is localized to the inner mitochondrial membrane where it accepts electrons from the FAD-dependent dehydrogenases of mitochondrial fatty acid β-oxidation as well as several dehydrogenases involved in amino acid metabolism. The function of ETFDH is to transfer the electrons from the ETF to ubiquinone (CoQ10) of the electron transport chain.
The lipid myopathies also include several of the diseases indicated above such as VLCAD, MCAD, and SCAD deficiencies, CPT-2 deficiency, and the mitochondrial trifunctional protein (MTP) deficiencies.
Other lipid myopathies have been shown to be the result of lipin 1 (phosphatidic acid phosphatase) deficiency, acyl-CoA dehydrogenase 9 (ACAD9) deficiency, and acetyl-CoA acyltransferase 2 (ACAA2; also known as medium-chain 3-ketoacyl-CoA thiolase, MCKAT) deficiency.
3-Hydroxyacyl-CoA Dehydrogenase Deficiency
The 3-hydroxyacyl-CoA dehydrogenase is an enzyme involved in medium- and short-chain fatty acid β-oxidation. This enzyme is also referred to as hydroxyacyl-CoA dehydrogenase and as short-chain L-3-hydroxyacyl-CoA dehydrogenase (SCHAD) and also as medium/short-chain L-3-hydroxyacyl-CoA. This enzyme is encoded by the HADH gene. This dehydrogenase exhibits highest specificity for medium-chain saturated fatty acids.
There can be confusion with the use of the acronym, SCHAD, since the enzyme encoded by the HSD17B10 gene, which is involved in the metabolism the branched-chain amino acid leucine, is also known as 3-hydroxy-2-methylbutyryl-CoA dehydrogenase, and as short chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD), and as 3-hydroxyacyl-CoA dehydrogenase type II.
Mutations in the HADH gene are associated with a disorder identified as familial hyperinsulinemic hypoglycemia, FHH. FHH is an autosomal recessive disorder first identified in 1991. Symptoms identified in the initial patient included juvenile-onset recurrent myoglobinuria, hypoketotic hypoglycemic encephalopathy, and hypertrophic/dilated cardiomyopathy.
Subsequently several cases of sudden infant death that were associated with hypotonia, hypoglycemia, hepatic steatosis, and hypoketotic dicarboxylic aciduria, were attributed to mutations in the HADH gene. In these latter clinical cases the descriptions defined HADH mutations with the family of related disorders referred to as persistent hyperinsulinemic hypoglycemia of infancy (PHHI).
Biochemically, disorders caused by HADH mutations are associated with elevated levels of 3-hydroxybutyryl-carnitine in the blood and elevated levels of 3-hydroxyglutaric acid in the urine.
Medium-Chain 3-Ketoacyl-CoA Thiolase Deficiency
As indicated in the Lipid Storage Myopathies section above, medium-chain 3-ketoacyl-CoA thiolase (MCKAT) is encoded by the ACAA2 (acetyl-CoA acyltransferase 2) gene. Mutations in the ACAA2 gene are extremely rare with only a single child in the United States having been identified with a mutant form of this enzyme. The infant died at 13 days which was associated with hypoglycemia, hyperammonemia, vomiting, dehydration, and myoglobinuria.
Peroxisomal Fatty Acid Oxidation Defects
Disorders related to the peroxisomes are divided into two categories. One category, exemplified by Zellweger syndrome, contains the disorders that result from defects in the biogenesis of the peroxisomes. The other category, exemplified by Refsum disease, contains the disorders that are due to mutations in a single peroxisomal enzyme.
Refsum disease is a rare autosomal recessive disorder in which patients harbor a defect in the peroxisomal α-oxidizing enzyme, phytanoyl-CoA hydroxylase (PhyH). Although mutations in PhyH are the primary cause of Refsum disease, the syndrome can also result from defects in the peroxisomal protein (PEX7) responsible for the import of PhyH into the peroxisome. Patients accumulate large quantities of phytanic acid in their tissues and serum. This leads to severe symptoms, including cerebellar ataxia, retinitis pigmentosa, nerve deafness and peripheral neuropathy. As expected, the restriction of dairy products and ruminant meat from the diet can ameliorate the symptoms of this disease. It should be noted that accumulation of phytanic acid is not solely the result of defects in PhyH.
Phytanic acid accumulation is also seen when there are inherited defects in peroxisome function leading to Zellweger syndrome, neonatal adrenoleukodystrophy and infantile Refsum disease. In addition, rhizomelic chondrodysplasia punctata, type 1 (RCDP1) results in phytanic acid accumulation. Refsum disease due to deficiency in PhyH is properly referred to as classical Refsum disease to distinguish it from infantile Refsum due to peroxisome dysfunction.
Mutations in the HSD17B4 gene are the cause of the autosomal recessive disorder referred to as D-bifunctional protein deficiency.