Last Updated: October 28, 2025
Introduction to Factor X
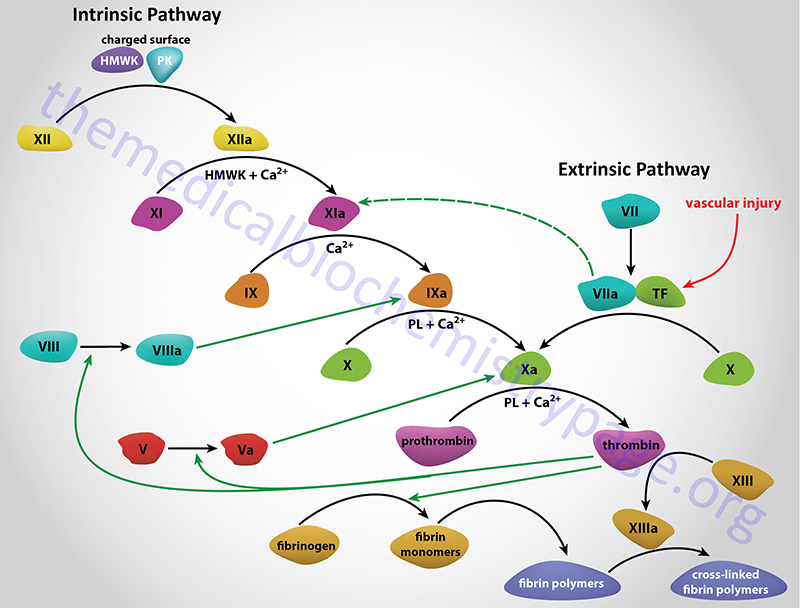
Factor X is historically identified as Stuart-Prower Factor. Factor X, along with prothrombin, factors VII, and IX, protein C, protein S, and protein Z, is modified by the vitamin K-dependent enzyme, γ-glutamyl carboxylase. This modification introduces a γ-carboxylate group into the amino acid glutamate generating what is referred to as a “gla” residue. These gla residues are critical for the interaction of these proteins with calcium (Ca2+) ions.
Factor X is activated to Xa in a complex termed the tenase complex. The tenase complex is composed of factor X, activated factor IX (IXa), phospholipids, and Ca2+. Following its activation factor Xa is incorporated into the prothrombinase complex which cleaves prothrombin to thrombin, thereby activating it.
Molecular Biology of Factor X
Factor X is encoded by the F10 gene. The F10 gene is located on chromosome 13q34 spanning 25 kbp and is composed of 8 exons that generate three alternatively spliced mRNAs, each of which encode a distinct precursor protein isoform.
Exon 1 encodes the leader peptide, exon 2 encodes the proprotein region cleaved by the “tenase complex” as well as the gla-residue-rich domain, exons 4 and 5 encode epidermal growth factor (EGF)-like domains, exon 6 encodes the activation domain, and exons 7 and 8 encode the catalytic domain.
Clinical Features fo Factor X Deficiency
Deficiency of the blood coagulation factor X (also called Stuart-Prower factor deficiency) is an autosomal recessive disorder that manifests with prolonged nasal and mucosal hemorrhage, menorrhagia, hematuria, and occasionally hemarthrosis (bleeding in the joints). The disorder gets its original name from the names of the first two individuals identified with this form of bleeding disorder, Mr. Stuart and Miss Prower. Deficiency of factor X in females can be particularly troublesome as it will result in adverse fetal outcomes such as recurrent spontaneous abortions, placental abruptions, and premature births.
The factor X protein is synthesized in the liver as a single polypeptide but is processed into a heavy chain and a light chain held together by disulfide bonds. As might be expected, due to the dependence of its serine protease activity on vitamin K-dependent carboxylation, factor X shows a high degree of homology to the other vitamin K-dependent factors of coagulation.