Last Updated: December 14, 2025
Introduction to the Muscular Dystrophies
The muscular dystrophies represent a group of nine characterized disorders, all of which are associated with some level of loss of muscle function along with atrophy of muscle tissue. These diseases are Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), myotonic dystrophy (DM, for dystrophia myotonica), distal muscular dystrophy, Emery-Dreifuss muscular dystrophy, limb-girdle muscular dystrophy, oculopharyngeal muscular dystrophy, fascioscapulohumeral muscular dystrophy, and congenital muscular dystrophy. Myotonic dystrophy results from a trinucleotide repeat expansion (CTG) present in the 3′-untranslated region of the dystrophia myotonica protein kinase gene (DMPK).
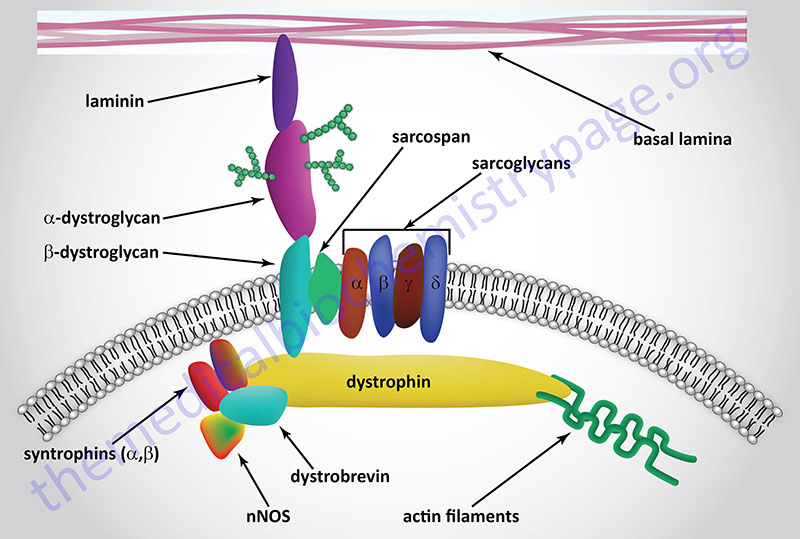
The muscular dystrophies that are associated with defects in the gene encoding the intracellular protein, dystrophin (gene symbol, DMD), are known as Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD). Given that the dystrophin gene is found on the X chromosome, both of these diseases are inherited in an X-linked manner. Both DMD and BMD are caused by mutations in the DMD gene and at least 65% of the mutations involve deletion of one or more of the 89 exons that comprise the DMD gene. The primary clinical differences between DMD and BMD are due to the fact that the mutations in the DMD gene that cause Duchene muscular dystrophy result in virtually no functional dystrophin protein being made. With Becker muscular dystrophy the mutations result in some functional dystrophin protein ranging from 10%–40% of the normal level of function.
Molecular Biology of Duchenne Muscular Dystrophy
The gene encoding dystrophin (DMD) is located on the X chromosome (Xp21.2–p21.1) and spans over 2 Mbp of DNA. The DMD gene is composed of 89 exons that generate 17 different mRNAs through the use of alternative promoters and through alternative splicing.
There are four Dp427 mRNAs generated from the DMD gene. The DMD mRNA that encodes the dystrophin isoform, Dp427m (dystrophin protein 427 kiloDalton muscle), is the main mRNA expressed in muscle. The Dp427m form of dystrophin is 3,685 amino acids in length. The DMD mRNA that encodes the dystrophin isoform, Dp427c, is predominantly expressed in neurons of the cortex and the dentate gyrus domain of the hippocampus.
The two Dp260 mRNAs initiate from a promoter and an exon 1 that reside within intron 29 and utilize exons 30-79.
The five Dp140 mRNAs initiate from a promoter and an exon 1 that reside within intron 44. These mRNAs utilize exons 45-79 with alternative splicing of exons 71, 72, 73, 74, and 79 generating the five Dp140 mRNAs..
The single Dp116 mRNA initiates from a promoter and an exon 1 that reside within intron 55 and uses exons 56-79.
The four Dp71 mRNAs use exons 63-79 with a novel 80 to 100 nucleotide exon containing an ATG start site. The different Dp71 mRNAs result from alternative splicing of exons 71 and 78.
The single Dp40 mRNA uses exons 63-70.
Duchenne muscular dystrophy is associated with a variety of disruptions in the DMD gene. The most common causes (65%) are deletions. Most of the deletions within the DMD gene in Duchenne muscular dystrophy are found between exons 45 and 53, referred to as a hotspot region. Gene duplications are found in 6%–10% of Duchenne muscular dystrophy patients. The remainder of cases involve small mutations (10%) or other smaller rearrangements. All of these disruptions interrupt the open reading frame of the DMD gene and prevent functional dystrophin protein from being synthesized.
The loss of dystrophin in the dystrophin-glycoprotein complex (DGC) results in membrane instability and increased susceptibility to fibrotic lesions resulting in the severe muscle wasting typical of the disease. This muscle wasting results in respiratory and cardiac failure and ultimately death, usually by the age of 30.
Because DMD is a lethal disorder it would be expected that the disease would eliminate itself due to lack of sexual transmission given that many males are too disabled by sexual maturity to pass on the mutant alleles. However, it has been found that approximately 25%-30% of all Duchenne muscular dystrophy patients arise due to new (de novo) mutations. If it is assumed that the incidence of a severe X-linked disease, such as Duchenne muscular dystrophy, does not change, then the mutation rate (identified by the Greek mu: μ) will equal the coefficient of selection (identified as “s”) multiplied by the mutant allele frequency (identified as q) divided by three (multiplied by one-third). The coefficient of selection represents the percentage of mutant alleles that are not inherited by the next generation. The one-third represents the fact that DMD only affects males and males carry 1/3 of the X chromosomes in any given population. With X-linked lethal diseases such as DMD, it is not possible to pass the mutant alleles to descendants; therefore, the coefficient of selection (s) is equal to 1. The mutation rate for DMD is, therefore, μ=[sq]/3, or μ=q/3. Given that it is assumed that one-third of mutated X alleles disappear in each generation, one-third of the identified mutations must occur as new mutations to take their place maintaining the relative population frequency, which in the case of Duchenne muscular dystrophy is 1 in 3,600 live births.
Clinical Features of Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) represents the most severe form of nine characterized muscular dystrophies. DMD is an X-linked recessive disorder and, therefore, primarily manifests in males. DMD is a rapidly progressing, fatal form of muscular dystrophy that is characterized by progressive muscle degeneration and weakness, eventually resulting in death. Symptoms of DMD usually begin to appear within the first 6-months of life but can also be seen at birth in some afflicted infants. The symptoms of DMD progress very quickly and usually the patients require the use of a wheelchair by the age of 10 years. The average life span for DMD patients is around 25 years of age.
The early signs that are characteristic in all DMD patients is a progressive proximal muscle weakness evident in the legs and pelvis. These symptoms are associated with, and the result of, a loss of muscle mass. Although muscle loss is characteristic of DMD, very early in the disease the calf muscles hypertrophy. As the disease progresses the muscle loss and weakness spreads to the arms, neck, and other areas of the body. As the disease progresses, the loss of muscle tissue is replaced fatty tissue and fibrotic tissue.
Therapeutic Intervention for Duchenne Muscular Dystrophy
The current standard therapy for patients with Duchenne muscular dystrophy is the use of corticosteroids. This therapy does not offer a cure but it does delay the progression of the muscle wasting. Although corticosteroids are the only pharmacologic agents with documented benefits, this therapy is associated with several adverse side-effects that includes weight gain, Cushingoid appearance, nervous system disturbance, gastrointestinal symptoms, metabolic disorders, and osteoporosis with increased risk of vertebral fractures. The mechanisms by which corticosteroids benefit patients with DMD is not yet completely understood.