Last Updated: January 20, 2026
G-Proteins
G-proteins are so-called because their activities are regulated by binding and hydrolyzing GTP. When a G-protein is bound to GTP it is in the active (“on”) state and when the GTP is hydrolyzed to GDP the protein is in the inactive (“off”) state. The G-proteins possess intrinsic GTPase activity that is regulated in conjunction with interaction with various forms of regulatory proteins. In many cases the regulation of a G-protein is exerted through its interaction with membrane-associated signal transducing receptors termed G-protein coupled receptors, GPCR.
There are two major classes of G-protein: those that are composed of three distinct subunits (α, β and γ), and are therefore referred to as heterotrimeric G-proteins, and the monomeric class that are related to the archetypal member Ras (originally identified as an oncogene causing sarcomas in rats). The monomeric class of G-protein is also referred to as the Ras superfamily or the small GTPase family of G-proteins. The structure and function of the monomeric G-proteins is similar to that of the α-subunit of the heterotrimeric G-proteins.
All known cell surface receptors that are of the G-protein coupled receptor class interact with heterotrimeric G-proteins. The α-subunit of the heterotrimeric class of G-proteins is responsible for the binding of GDP/GTP. When G-proteins are activated by receptors or intracellular effector proteins there is an exchange of GDP for GTP turning on the G-protein which enables it to transmit the original activating signal to downstream effector proteins. In the heterotrimeric class of G-protein, when associated receptor activation stimulates the GDP for GTP exchange in the α-subunit, the protein complex dissociates into separate α and βγ activated complexes. The released and activated βγ complex serves as a docking site for interaction with downstream effectors of the signal transduction cascade or as direct activators. Once the α-subunit hydrolyzes the bound GTP to GDP it re-associates with the βγ complex thereby terminating its activity.
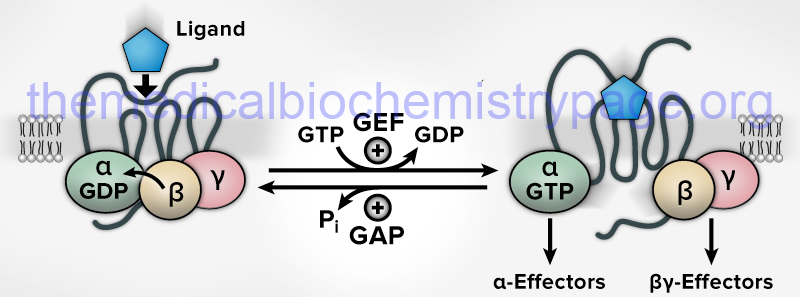
The GTPase activity of G-proteins is augmented by GTPase activating proteins (GAP) and the GDP/GTP exchange reaction is catalyzed by guanine nucleotide exchange factor (GEF) activity. As indicated in the above Figure, the GEF activity for the majority of the heterotrimeric G-proteins is an intrinsic activity of their associated GPCR. Exceptions to this include the translation factor, eIF-2B, which is the GEF activity for the α-subunit of the heterotrimeric G-protein translation initiation factor, eIF-2. For the monomeric G-proteins there are distinct GEF proteins.
The major difference between the GEF activity of GPCR and the GEF activity of distinct enzymes is that the latter have fully reversible activity such that they can also bind to GTP-bound G-proteins and catalyze the exchange of GTP for GDP. Within the Rho and Rab small GTPase family of G-proteins there are guanine nucleotide dissociation inhibitors (GDIs) that maintain the G-protein in its inactive GDP-bound state.
Gα Subtypes and Functions
The Gα subunits are a family of 39–52 kDa proteins that share 45%–80% amino acid similarity. There are at least 16 Gα subunit genes in the human genome, with several genes expressing splice variants. The distinction of the different types of α-subunits found in heterotrimeric G-proteins is based upon the downstream signaling responses activated or inhibited as a result of G-protein activation. These classifications allow the Gα subunits to be divided into four subtypes include Gs (since it is the α-subunit these designations are also written Gαs), Gi, Gq, and G12.
The Gαs Family
The Gs family of G-proteins is comprised of Gαs and Gαolf. These α-subunits stimulate the activity of adenylate cyclase resulting in increased production of cAMP from ATP. Increased production of cAMP results in the activation of PKA. Gαolf is so-called due to the fact that it was originally identified as being involved in olfaction.
The Gαs protein is encoded by the GNAS locus. The GNAS locus is located on chromosome 20q13.32 and is composed of 22 exons. The GNAS locus exhibits a complex pattern of expression being controlled by imprinting and four alternative promoters. The GNAS locus controls maternally, paternally, and biallelically derived mRNAs and also exerts added complexity via alternative splicing such that ten different protein isoforms result. These different Gαs isoforms are identified as GNASL, GNASS, GNASXlas, GNASf, GNASg, GNASh, ALEX (GNASXL), ALEXh, ALEXi, and SCG6.
The Gαolf protein is encoded by the GNAL gene. The GNAL gene is located on chromosome 18p11.21 and is composed of 17 exons that generate five alternatively spliced mRNAs that collectively encode three distinct protein isoforms.
The Gαi Family
The Gi class is comprised of Gαi, Gαo, Gαz, Gαt, and Gαgust. These α-subunits either inhibit adenylate cyclase thereby, inhibiting the production of cAMP (Gαi, Gαo, Gαz) or activate phosphodiesterases leading to increased hydrolysis of cGMP (Gαt) or cAMP (Gαgust).
The βγ subunits that are associated with Gαi and Gαo function to open membrane K+ channels of the large family of inwardly-rectifying K+ channels.
The designation Gαt defines this α-subunit as being in transducin which is the G-protein activated by the visual receptor rhodopsin.
The designation Gαgust defines this α-subunit as being in gustducin which is a G-protein involved in the gustatory system which is the sensory system for taste.
Within the Gαi family there are three distinct Gi encoding genes (GNAI1, GNAI2, and GNAI3), one Gαo gene (GNAO1), three Gαt genes (GNAT1, GNAT2, and GNAT3), and one Gαz gene (GNAZ).
The Gαq Family
The Gq class (comprised of Gαq, Gα11, Gα14, and Gα15) activates membrane-associated PLCβ resulting in increased production of the intracellular messengers IP3 and DAG. This class of G-protein is associated with adrenergic (specifically α1-adrenergic), muscarinic, serotonin, and histamine receptors. The four genes in this family are GNAQ, GNA11, GNA14, and GNA15.
The Gα12 Family
The G12 family is comprised of Gα12 and Gα13. The Gα12 family of G-proteins, encoded by the GNA12 and GNA13 genes, is involved in the activation of the Rho family of monomeric G-proteins.
Gβγ Subtypes and Functions
The primary function that was originally proposed for the βγ-subunits (Gβγ dimer) was solely that of an inactivator of Gα subunits. As inactivators of Gα subunits, the Gβγ dimer was proposed to facilitate the re-association of the inactive heterotrimer with the receptor for subsequent rounds of signaling. In this capacity it was viewed that the Gβγ dimer was a negative regulator of Gα signaling. However, in 1987 it was shown that Gβγ dimers were able to activate a cardiac inwardly rectifying potassium channel normally activated by acetylcholine.
Subsequent to this initial observation it was found that the Gβγ subunits could modulate many other effectors via direct interactions. Indeed, many of the effectors are those that are also regulated by Gα subunits such as phospholipase Cβ (PLCβ), several adenylate cyclase (AC) isoforms, phosphoinositide-3 kinases (PI3K), and voltage-gated calcium channels.
In addition to these membrane-associated targets activated by Gβγ subunits, the dimers have also been shown to effect modulation of numerous other proteins located throughout the cell. These include proteins in the cytosol, nucleus, endosomes, mitochondria, ER, Golgi apparatus, and cytoskeleton. It is, however, not fully understood as yet, whether all of these intracellular events require an initial event triggered through a GPCR and/or whether Gα subunits are also involved in the overall Gβγ activation process.
A total of five Gβ (β1–5) genes and twelve Gγ (γ1–5, 7–13) are genes expressed in humans. The human Gβ subunit genes are identified as GNB1–GNB5. The human Gγ subunit genes are identified as GNGT1 and GNGT2 (which encode the Gγ subunits of transducin, Gt), GNG2–GNG5, GNG7, GNG8, GNG10–GNG14.
The Gβ1-4 subunits share extensive amino acid sequence homology of between 79% and 90%. The Gβ5 subunit is only approximately 52% identical to the other four Gβ subunits. In addition, there are two Gβ3 isoforms (β3 and β3S) and two Gβ5 isoforms (β5 and β5L). It is likely that the Gβ subunit genes evolved from a common ancestor into two subfamilies with one consisting of the Gβ1-4 subtypes and another consisting of Gβ5 subtypes.
The twelve known Gγ subunits are much more diverse, exhibiting amino acid similarities of between 26% and 76%. The Gγ subunit genes diverged from each other into five classes designated I through V. The class I group includes Gγ7 and Gγ12. Class II contains Gγ2, Gγ3, Gγ4, and Gγ8. Class III contains Gγ5 and Gγ10. Class IV contains Gγ1, Gγ9, and Gγ11. Class V contains Gγ13. Given the large diversity in resultant Gβγ dimer composition it is very likely that widely varied functional roles for the various dimers also exists.
Although the canonical adenylate cyclase (AC) enzyme has long been known to be activated by Gαs-type G-proteins and inhibited by the Gαi-type G-proteins, there are several known isoforms of AC in human tissues. All of the AC isoforms are transmembrane proteins consisting of two sets of six transmembrane segments separated by cytoplasmic (C1 and C2) domains. Certain isoforms of AC are regulated by direct interaction with Gβγ subunits. The consequences of Gβγ binding is isoform specific with some forms being activated and others inhibited by the interaction with Gβγ dimers. In all of the AC isoforms activated by Gβγ dimers (AC2, AC4, and AC7), the site of interaction has been shown to contain a motif consisting of the amino acids: PFAHL. This motif is absent in the AC isoforms that are not activated by Gβγ dimers.
There are 13 phospholipase C (PLC) genes in the human genome and the first subfamily demonstrated to be regulated by Gβγ was PLCβ. Indeed, each of the four PLCβ isoforms is regulated by both Gαq and Gβγ resulting in increased phospholipase activity. However, the regulation involves distinct binding sites on the PLCβ protein. The binding of Gαq occurs within a domain at the C-terminus whereas the Gβγ dimers bind to a domain at the N-terminus. In addition to the PLCβ isoforms, the Gβγ dimers regulate the activities of the PLC epsilon (PLCε) and PLC eta (PLCη) isoforms.
Several members of the large voltage-gated potassium subfamily J channels family of channels, commonly referred to as inwardly-rectifying potassium channels (family member proteins identified as Kir) are activated through their association with GPCR signaling cascades. These receptor-coupled Kir potassium channels are the Kir3 channels of which there are four identified as Kir3.1 (encoded by the KCNJ3 gene), Kir3.2 (encoded by the KCNJ6 gene), Kir3.3 (encoded by the KCNJ9 gene), and Kir3.4 (encoded by the KCNJ5 gene). Because of the activation of these four potassium channels due to direct association with GPCR mediated signaling they are also known as G-protein coupled inwardly-rectifying K+ channels, GIRK. The KCNJ3 gene encodes GIRK1, KCNJ6 encodes GIRK2, KCNJ9 encodes GIRK3, and KCNJ5 encodes GIRK4. All four of the GIRK channels are activated by direct binding of Gβγ dimers. The KCNJ3 and KCNJ5 encoded proteins form a heterotetrameric channel associated with the cardiac muscarinic acetylcholine receptor, specifically the M2 receptor.
Voltage-dependent Ca2+ channels (Cav) are responsible for calcium ion flux across plasma membranes. The main pore-forming protein of these channels, the α1 subunit, is classified into three groups: Cav1, Cav2, and Cav3. In addition to the pore-forming α-subunits, Cav channels contain a cytoplasmic Cavβ subunit and a membrane-associated α2/δ subunit. The α1 subunits harbor the Gβγ binding sites and all three classes of channel are regulated by Gβγ dimer binding.
Monomeric G-proteins
The RAS superfamily of small monomeric G-proteins comprises well over 100 different proteins. This superfamily is divided into eight main families. The eight major families are RAS (31 members), RAS-related (4 members), RHO (20 members), RAB (64 members), RAB-like (6 members), RAN (1 member), ARF (31 members), and MIRO (for mitochondrial RHO; 2 members).
The RAS family is primarily responsible for regulation of events of cell proliferation. The RHO family is involved in the regulation of cell morphology through control of cytoskeletal dynamics. The RAB family is involved in membrane trafficking events. The RAN protein is involved in regulation of nuclear transport. The ARF family is involved in intracellular vesicle transport.
The RHEB protein, which belongs to the RAS family, gets its name from RAS homolog expressed in brain. The RHEB protein is involved in the regulation of mTOR (mammalian target of rapamycin).
G-Protein Regulators
The activity state of G-proteins is regulated both by the rate of GTP exchange for GDP and by the rate at which the GTP is hydrolyzed to GDP. The GTP for GDP exchange process is catalyzed by guanine nucleotide exchange factor (GEF) activity. With respect to GPCR, the receptor itself has intrinsic GEF activity that is activated upon ligand binding. The activity of G-proteins with respect to GTP hydrolysis is regulated by a family of proteins termed GTPase activating proteins, GAP. Both of these G-protein regulatory protein classes are classified as regulators of G-protein signaling, RGS. The GAP proteins that act on the Gα subunits of the GPCR linked heterotrimeric G-proteins are all encoded by genes identified as RGS genes. Humans express a total of 21 genes in the RGS family identified as RGS1–RGS14, RGS16–RGS22.
In addition to the RGS gene encoded proteins there are several proteins that contain RGS domains such as the G-protein coupled receptor kinases, GRK1, GRK4, GRK5, GRK6, and GRK7. There are also numerous GAP proteins that regulate the activity of the various members of the monomeric G-protein superfamily. For example there are 50 genes in the human genome that express Rho GAP proteins. The translation initiation factor eIF-5 is another member of the large GAP family of proteins. Another related family of proteins are termed the RGS-like proteins.
The proto-oncogenic protein, Ras, is a G-protein involved in the genesis of numerous forms of cancer (when the protein sustains specific mutations). Of particular clinical significance is the fact that oncogenic activation of Ras occurs with higher frequency than any other gene in the development of colorectal cancers. Regulation of Ras GTPase activity is controlled by RasGAP (encoded by the gene). There are several other GAP proteins besides RasGAP that are important in signal transduction. There are two clinically important proteins of the GAP family of proteins. One is the gene product of the neurofibromatosis type-1 (NF1) susceptibility locus. The NF1 gene is a tumor suppressor gene and the protein encoded is called neurofibromin. The second is the protein encoded by the BCR locus (break point cluster region gene). The BCR locus is rearranged in chronic myelogenous leukemias (CML) harboring the Philadelphia positive (Ph+) chromosome and in certain acute lymphocytic leukemias (ALL).
G-Protein Coupled Receptors: GPCR
There are several different classifications of receptors that couple signal transduction to G-proteins. These classes of receptor are termed G-protein coupled receptors, GPCR. All GPCR are composed of a similar structure that includes seven membrane-spanning helices connected by three intracellular loops and three extracellular loops with an extracellular amino terminus and an intracellular carboxy terminus.
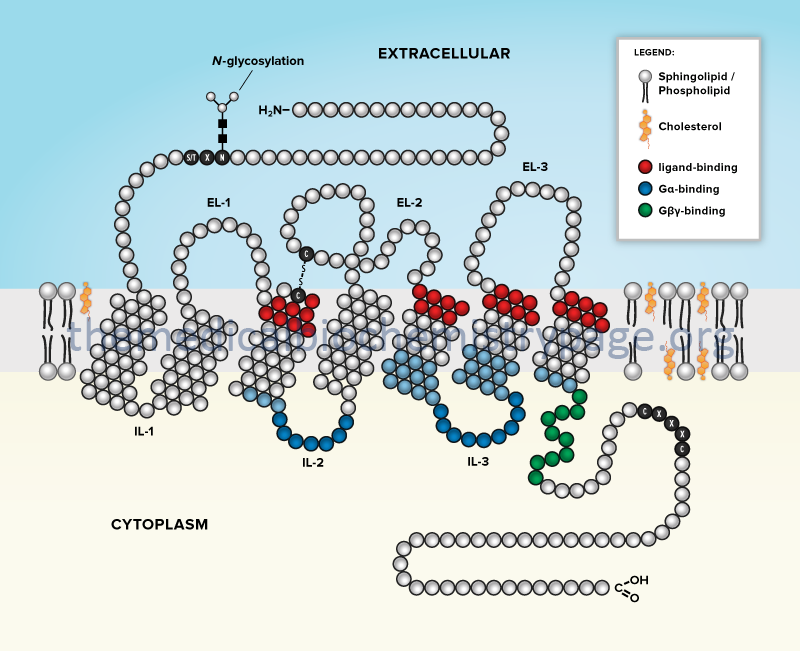
The GCPR superfamily of transmembrane proteins all belong to the type IV-B multipass transmembrane protein family. There are at least 1409 identified GPCR genes in the human genome, although not all are protein coding as several hundred are pseudogenes. In addition, many of the protein coding GPCR genes encode proteins for whom the ligand(s) is unknown and are, therefore, referred to as orphan GPCR.
The GPCR superfamily consists of six defined families or classes. These six families are the class A rhodopsin-like receptors, the class B secretin-like receptors, the class C metabotropic glutamate/pheromone receptors, the class F frizzled receptors, the taste receptors, and the vomeronasal (chemosensory system detecting pheromones) receptors.
The vast majority of G-proteins to which GPCR are coupled are members of the heterotrimeric G-protein family discussed in the G-Proteins section above. All trimeric G-proteins, whether or not they are coupled to receptor-mediated signal transduction cascades, are composed of three subunits: α, β, and γ. The α-subunit is responsible for the activity of the G-protein and the βγ subunits are regulatory and are involved in binding GTP. All GPCR act as guanine nucleotide exchange factors (GEFs) and when they are activated by ligand binding, they catalyze exchange of GDP, tightly bound to the α-subunit of heterotrimeric G-proteins, for GTP.
Class A GPCR Superfamily
The class A GPCR superfamily is referred to as the rhodopsin-like family. Class A contains the largest number of members compiled into at least 21 classes (families). Class A GPCRs include opsins, the vast majority of the odorant (olfactory) receptors, and receptors for monoamines, purines, opioids, chemokines, some small peptide hormones, and the large glycoprotein hormones that consist of thyroid stimulating hormone (TSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH).
Table of Class A GPCR Proteins
| Class A Superfamily | Family Members/Genes | Comments |
| Amine receptors | six subfamilies of receptors | collectively includes 45 genes (4 of which are pseudogenes); the six subfamilies are the 5-hydroxytryptamine (5-HT, serotonin) receptors (13 genes), the dopamine receptors (5 genes), the histamine receptors (4 genes), the muscarinic acetylcholine (mAChR) receptors (5 genes), the adreno receptors (9 genes), and the trace amine receptors (9 genes, 3 of which are pseudogenes) |
| Chemokine receptors | five subfamilies of receptors | CXC motif receptors: six family member genes CXCR1–CXCR6 CC motif receptors: ten family member genes CCR1–CCR10 CX3C motif receptor: CX3CR1 XC motif receptor: XCR1 Atypical chemokine receptors: ACKR1, ACKR2, ACKR3, ACKR4, CCRL2, PITPNM3 |
| Olfactory receptors | 18 subfamilies | is a superfamily of receptors that is composed of subfamilies 1–14, 51, 52, 55, and 56; collectively there are 861 identified genes in olfactory receptor family, many are suspected to be pseudogenes or are in fact pseudogenes; all of the olfactory family 51, 52, 55, and 56 receptors are located on chromosome 11p15.4 |
| Complement component receptors | C3AR1, C5AR1, C5AR2 | C3AR1 encodes the receptor for complement system component C3a; C5AR1 and C5AR2 encode receptors for complement system component C5a |
| Formyl peptide receptors | FPR1, FPR2, FPR3 | chemotaxis receptors that bind N-formylated peptides derived from the degradation of bacteria |
| Glycoprotein hormone receptors | FSHR, LHCGR, TSHR | see the Peptide Hormones and Their Receptors page for more detailed information on the glycoprotein hormones and their receptors |
| Hydroxycarboxylic acid receptors | HCAR1, HCAR2, HCAR3 | HCAR1 encoded protein is HCA1 and is also known as GPR81; receptor for lactate HCAR2 encoded protein is HCA2 and also is known as GPR109A HCAR3 encoded proteins is HCA3 and is also known as GPR109B details on the hydroxycarboxylic acid receptors are in the Bioactive Lipids and Lipid Sensing Receptors page |
| Lipid-like receptors | seven subfamilies of receptors | cannabinoid receptors: CNR1 gene encodes the CB1 protein, CNR2 encodes the CB2 protein Free fatty acid receptors: FFAR1 (GPR40), FFAR2 (GPR43), FFAR3 (GPR41), FFAR4 (GPR120) Leukotriene receptors: CYSLTR1, CYSLTR2, FPR2, LTB4R, LTB4R2, OXER1 Prostaglandin receptors: PTGDR, PTGDR2, PTGER1, PTGER2, PTGER3, PTGER4, PTGFR, PTGIR, TBXA2R Sphingosine-1-phosphate (S1P) receptors: S1PR1–S1PR5 Lysophosphatidic acid (LPA) receptors: LPAR1–LPAR6 Platelet activating factor (PAF) receptor: PTAFR |
| Melatonin receptors | MTNR1A, MTNR1B | melatonin is derived from the amino acid tryptophan and its synthesis and functions are discussed in the Amino Acid Derivatives: Neurotransmitters, Nitric Oxide, and More page |
| Nucleotide-like receptors | adenosine receptors: A1 (ADORA1), A2A2B (ADORA2B), A3 (ADORA3) P2Y receptors: P2RY1, P2RY2, P2RY4, P2RY6, P2RY11–P2RY14 | adenosine acts as an inhibitory neurotransmitter in the CNS; exerts effects in the periphery on the heart and the lungs, and also exerts anti-inflammatory effect; A1 and A3 are coupled to Gi-type G-proteins; A2A is coupled to a Gs-type G-protein; A2B is coupled to both Gs– and Gq-type G-proteins; activation of A1 results in bronchoconstriction and decreased heart rate; activation of A2A results in reduced neural excitation in the CNS, decreased dopaminergic stimulation, and coronary artery vasodilation; activation of A2B results in bronchoconstriction; activation of A3 relaxes cardiac muscle, causes smooth muscle contraction, and inhibits neutrophil degranulation the P2Y receptors are discussed in the Signal Transduction Pathways: Nucleotides page |
| Opsin receptors | RHO, OPN1LW, OPN1MW, OPN1MW2, OPN1MW3, OPN1SW, OPN3, OPN4, OPN5, RGR, RRH | all opsins are light-sensitive GPCR with rhodopsin (encoded by the RHO gene) being the light receptor in rod cells in the retina; the opsins (OPN genes) are primarily localized to the cone cells in the retina |
| Prokineticin receptors | PROKR1, PROKR2 | prokineticins are gastrointestinal (GI) hormones that act to induce smooth muscle contraction in the GI; these hormones are also involved in nociception (sensation of pain), angiogenesis, and neurogenesis |
| Relaxin family peptide receptors | RXFP1, RXFP2, RXFP3, RXFP4 | the relaxins are a family of peptide hormones that belong to the insulin superfamily of hormones; these hormones are structurally most closely related to the insulin-like (INSL) peptides |
| Chemerin receptors | CMKLR1, CMKLR2 | chemerin is an immune system modulating cytokine produced and secreted by adipose tissue; regulates adipocyte development and exerts effects on glucose metabolism in both the liver and skeletal muscle |
| F2R receptors | F2R, F2RL1, F2RL2, F2RL3 | these genes encode the protease-activated receptor (PAR) family proteins; F2R encoded protein is PAR1 which is a receptor for the coagulation factor thrombin; F2RL1 encodes PAR2, F2RL2 encodes PAR3, F2RL3 encodes PAR4 |
| Peptide receptors | 20 subfamilies of receptors | this superfamily represents the receptors for the opioids, angiotensin, bradykinin, cholecystokinin, somatostatin, oxytocin and arginine vasopressin, melanocortins, melanin-concentrating hormone, endothelins, galanin, gonadotropin releasing hormone, orexins, apelin, tachykinins, neurotensins, neuromedin U, and the neuropeptides; the neuropeptides include neuropeptide Y (NPY), neuropeptide B and W (NPB and NPW), neuropeptide S, and neuropeptide FF (NPFF) |
| G-protein coupled bile acid receptor | GPBAR1 | originally identified as TGR5 and is also known as GPR131; bile acids also bind and activate the farnesoid X receptors (FXR) which belong to the nuclear receptor superfamily |
| G-protein coupled estrogen receptor | GPER1 | estrogens predominantly bind to receptors that are members of the nuclear receptor superfamily; this receptor binds the estrogen, 17-β-estradiol (E2); activation of GPER1 results in modulation of cardiovascular, endocrine, reproductive, immune, and central nervous system functions |
| Oxoglutarate receptor | OXGR1 | is also known as the cysteinyl leukotriene receptor E (CysLTE); originally identified as GPR99; the receptor is also activated by leukotriene E4 (LTE4); receptor is closely related to the P2Y family of purinergic receptors; the drug montelucast, which is used to treat asthma, allergic rhinitis, and urticaria (hives), was originally developed for inhibition of the cysteinyl leukotriene receptor 1 (CysLT1) has been shown to inhibit the activation of OXGR1 as well, |
| Succinate receptor | SUCNR1 | was originally identified as GPR91; related to the P2Y family of purinergic receptors; is expressed in various blood cells, in adipose tissue, liver, retina, and kidney; mediates cellular responses to ischemia, hypoxia, toxicity, and hyperglycemia |
| Class A orphan GPCRs | large subfamily composed of 78 protein coding genes and 1 pseudogene | several members have been de-orphanized MAS1 protooncogene which is a Gq-coupled receptor for angiotensin(1-7); also includes nine MAS related proteins includes the proton-sensing (pH-sensing) receptors GPR4, GPR65 (TDAG8 for T-cell Death-Associated Gene 8), GPR68 (OGR1 for Ovarian cancer G protein-coupled Receptor 1), and GPR132 (G2A for G2 accumulating); GPR132 is also a receptor for lactate GPR34 is a receptor for lysophosphatidylserine (lysoPS) so is also identified as LPS1 GPR35 was originally shown to be activated by kynurenic acid which is an intermediate in tryptophan catabolism, also shown to be the receptor for the chemokine (C-X-C motif) identified as CXCL17 and as such it has been suggested that GPR35 be identified as CXCR8 (CXC motif chemokine receptor 8) GPR55 has been shown to be activated by 2-arachidonoyl lysophosphatidylinositol (2-ALPI) and palmitoylethanolamide (PEA) GPR75 is the receptor for ω-hydroxylated form of arachidonic acid, 20-hydroxyeicosatetraenoic acid (20-HETE) GPR84 is a receptor for medium-chain free fatty acids such as capric acid (C10:0), undecanoic acid (C11:0), and lauric acid (C12:0) GPR119 is the receptor for oleoylethanolamide (OEA) |
Class B GPCR Superfamily
The class B GPCR superfamily is referred to as the secretin-like receptor class. Class B is comprised of 6 classes (families) that include the receptors for calcitonin, parathyroid hormone, corticotropin releasing hormone, glucagon, and vasoactive intestinal peptide in addition to the large adhesion receptors family. The adhesion receptor family contains nine subfamilies identified as A, B, C, D, E, F, G, L, and V.
Table of Class B GPCR Proteins
| Class B Superfamily | Family Members/Genes | Comments |
| Adhesion receptors | 9 adhesion receptor subfamilies | adhesion receptor subfamily A: ADGRA1, ADGRA2, ADGRA3 adhesion receptor subfamily B: ADGRB1, ADGRB2, ADGRB3 adhesion receptor subfamily C (cadherin receptors): CELSR1, CELSR2, CELSR3 adhesion receptor subfamily D: ADGRD1, ADGRD2 adhesion receptor subfamily E: ADGRE1, ADGRE2, ADGRE3, ADGRE5 adhesion receptor subfamily F: ADGRF1–ADGRF5 adhesion receptor subfamily G: ADGRG1–ADGRG7 adhesion receptor subfamily L: ADGRL1–ADGRL4 adhesion receptor subfamily V: ADGRV1 |
| Calcitonin receptors | CALCR, CALCRL | see the Peptide Hormones page |
| Corticotropin releasing hormone (CRH) receptors | CRHR1, CRHR2 | see the Gut-Brain Interrelationships page |
| Glucagon receptors | GCGR, GHRHR, GIPR, GLP1R, GLP2R, SCTR | GCGR encodes the glucagon receptor GHRHR encodes the growth hormone releasing hormone (GHRH) receptor GIPR encodes the glucose-dependent insulinotropic peptide (GIP) receptor; GIP also called gastric inhibitory peptide GLP1R encodes the glucagon-like peptide 1 (GLP-1) receptor GLP2R encodes the glucagon-like peptide 2 (GLP-2) receptor SCTR encodes the secretin receptor |
| Parathyroid hormone receptors | PTH1R, PTH2R | see the Peptide Hormones page |
| Vasoactive intestinal peptide (VIP) receptors | VIPR1, VIPR2, ADCYAP1R1 | VIPR1 and VIPR2 encode the two receptors for vasoactive intestinal peptide (VIP); VIPR2 is 100% identical to VIPR1 except that it has a novel 67 amino acid N-terminus; VIP is produced in the gut and in the hypothalamus; VIP functions to induce smooth muscle relaxation leading to reductions in blood pressure, also stimulates cardiac contractility; within the gut VIP inhibits parietal cell acid production and chief cell release pf pepsinogen ADCYAP1R1 encodes the type 1 (pituitary) adenylate cyclase activating polypeptide (PACAP) receptor; PACAP is encoded by the ADCYAP1 gene; PACAP can also bind to the VIPR1 and VIPR2 encoded receptors; PACAP is a hormone that induces activity in the pituitary and also functions as a neurotransmitter |
Class C GPCR Superfamily
The class C superfamily is referred to as the metabotropic glutamate/pheromone receptor family. Class C is comprised of 4 classes (families) that include the metabotropic glutamate receptors (mGluR), extracellular Ca2+-sensing receptors, the GABA-B type receptors, as well as the class C orphan receptors.
Table of Class C GPCR Proteins
| Class C Superfamily | Family Members/Genes | Comments |
| Calcium sensing receptors | CASR, GPRC6A | the CASR encoded protein (identified as CaSR) is expressed in the parathyroid glands, renal tubule cells, bone marrow, thyroid gland C-cells, gastrin-secreting cells in the stomach, several areas of the brain, as well as in several other tissues; CaSR was the first receptor shown to be activated by an ion ligand the GPRC6A gene encodes a receptor for amino acids (preferably basic amino acids) as well as extracellular Ca2+ and the protein osteocalcin; osteocalcin is produced exclusively by osteoblasts and its function is regulated by vitamin K-dependent carboxylation |
| Gamma aminobutyrate type B (GABA-B) receptors | GABBR1, GABBR2 | the GABA-B receptors are metabotropic GABA receptors that are coupled to the activation of K+ channels; for more details see the Biochemistry of Nerve Transmission page |
| Metabotropic glutamate receptors | GRM1–GRM8 | see the Biochemistry of Nerve Transmission page |
| Class C orphan GPCRs | GPRC5A–GPRC5D, GPR156, GPR158, GPR179 | GPR156 is involved in maintaining auditory function GPR179 functions in retina in the transmission of signals from photoreceptors to on-bipolar cells; mutations in gene are one of the causes of complete congenital stationary night blindness (CSNB1E) |
Other Members of the GPCR Superfamily
Frizzled Receptors
The Frizzled receptor subfamily of GPCR is composed of 11 proteins encoded by the smoothened gene (SMO) and the FZD1–FZD10 genes. The SMO gene is also referred to as FZD11. This GPCR subfamily is atypical in that the receptors to not couple to canonical G-protein signaling pathways.
Taste Receptors
The taste (gustatory) receptor family is divided into the taste 1 receptor (TAS1R) and taste 2 receptor (TAS2R) subfamilies. There are three taste 1 receptor family members encoded by the TAS1R1–TAS1R3 genes. The TAS1R1 encoded receptor was originally identified as GPR70 and the TAS1R2 encoded receptor was originally identified as GPR71.
There are 39 genes of the taste 2 receptor family, 27 of which encode functional proteins, 11 of which are predicted to encode pseudogenes, and one of which is, as yet, undetermined to be coding or pseudogenes. Most of the functional TAS2R genes are localized to chromosome 7q34 or to chromosome 12p13.2.
The TAS1R encoded receptors are responsible for the sensation of sweet taste in the oral cavity, whereas the TAS2R encoded receptor are responsible for the sensation of bitter taste in the oral cavity. For example, caffeine, which is a bitter alkaloid in coffee activates several receptors of the TAS2R family including TAS2R7, TAS2R10, TAS2R14, TAS2R43, and TAS2R46.
The taste receptors are expressed on numerous cell types outside the oral cavity. For example receptors of both the TAS1R and TAS2R families are expressed in the gastrointestinal system where they are involved in processes such as gastric acid secretion, nutrient sensing, and innate immune functions. In addition to the gastrointestinal system, taste receptors are expressed in airway epithelial cells and the brain.
The vomeronasal (pheromone) receptor family is composed of 129 genes with only four (VN1R1, VN1R2, VN1R3, and VN1R4) encoding functional proteins.
GPCR Desensitization: Kinases and Arrestins
A characteristic feature of GPCR activity following ligand binding is a progressive loss of receptor-mediated signal transduction. This process is referred to as desensitization or adaptation. The events that reflect desensitization of a G-protein coupled signaling system can involve the receptor itself, the G-protein associated with the receptor, and/or the downstream effector(s).
In the majority of cases it is impairment of the receptor’s ability to activate its G-protein that accounts for most desensitization, especially within minutes of agonist stimulation. Within milliseconds to minutes of ligand binding, cells can diminish or virtually eliminate the receptor-mediated responses. This process involves phosphorylation of the GPCR on one or more intracellular domains. On a longer time scale (several hours after ligand binding) the short-term desensitization is augmented by receptor down-regulation which involves the loss of membrane-associated receptor through a combination of protein degradation, transcriptional, and posttranscriptional mechanisms.
GPCR Kinases
Heterologous desensitization involves phosphorylation of GPCR by second-messenger-dependent kinases, such as PKA and PKC. Receptor phosphorylation by these kinases, as an isolated event, substantially impairs the ability of GPCR to stimulate their G-proteins.
Homologous desensitization of GPCR involves a family of kinases termed G-protein coupled receptor kinases (GRK). The GRK constitute a family of six mammalian serine/threonine kinases that phosphorylate ligand-activated GPCR as their primary substrates, hence the designation of the process as homologous desensitization. These six kinases are identified as GRK1 (originally called rhodopsin kinase); GRK2 (originally called β-adrenergic receptor kinase-1, βARK1); GRK3 (originally called β-adrenergic receptor kinase-2, βARK2); GRK4 (originally called IT-11); GRK5; and GRK6.
Expression of GRK1 is almost exclusive to the retina and GRK4 expression is observed at significant levels only in testes. The remaining GRK are found ubiquitously expressed. These kinases preferentially phosphorylate ligand (agonist) bound and activated receptor rather than inactive or antagonist-occupied GPCR substrates. Interaction of GRK with their activated receptor substrates potently activates these enzymes. GRK-mediated GPCR phosphorylation requires the participation of regulatory mechanisms responsible for the membrane localization and receptor targeting of these enzymes.
The discovery of the GRK was the result of experiments designed to understand the mechanisms responsible for short-term, homologous desensitization of the β2-adrenergic receptor (β2AR) and rhodopsin. With respect to the β2AR, agonist-induced receptor phosphorylation associated with homologous desensitization was found to occur even in cells genetically lacking PKA. The enzyme responsible for this activity was subsequently purified and named β-adrenergic receptor kinase (now GRK1). Rhodopsin kinase (GRK1) was identified as the enzyme responsible for phosphorylating light bleached (agonist-activated) rhodopsin in rod outer segments. Subsequently, GRK1-mediated phosphorylation of rhodopsin was associated with desensitization of the rhodopsin/Gt/cGMP phosphodiesterase system. The GRK family of serine/threonine kinases shares the unusual feature of phosphorylating specifically the agonist-occupied, or activated, conformation of GPCRs.
GPCR Arrestins
The model proposed to describe the mode of action of GRK suggests that a receptor phosphorylated by a GRK can subsequently bind stoichiometrically to one of a family of cytoplasmic inhibitory proteins that have been termed the arrestins. Humans express four genes that encode arrestin protein family members.
In the rhodopsin system this inhibitory protein was initially referred to as arrestin but is now referred to as S-antigen visual arrestin (encoded by the SAG gene). In non-retinal tissues there are three related inhibitory proteins known as β-arrestin-1 (encoded by the ARRB1 gene), β-arrestin-2 (encoded by the ARRB2 gene), and arrestin 3 (encoded by the ARR3 gene). The SAG and ARR3 encoded proteins are highly expressed in the visual system while both of the β-arrestin proteins are found in most other tissues.
There can be confusion with respect to the nomenclature of the arrestin proteins since S-antigen visual arrestin (the originally characterized arrestin) is also known as arrestin-1, β-arrestin-1 is also referred to arrestin-2, β-arrestin-2 is also known as arrestin-3, and arrestin 3 is also known as arrestin-4.
The ability of an arrestin protein to recognize an activated and phosphorylated GPCR in an appropriate lipid environment is due to clusters of Arg and Lys residues in the N-terminal domain of the arrestin proteins. As a result of arrestin or β-arrestin binding, a GPCR is prevented from activating its G-protein and, therefore, its downstream effector(s). This two-step process of GRK-initiated desensitization can reduce by as much as 70%–80% the ability of fully activated β-adrenergic receptors or rhodopsin to activate their respective G-proteins.
Furthermore, the binding of β-arrestins to GRK-phosphorylated GPCR is believed to initiate GPCR endocytosis, or sequestration, into recycling endosomes. Within the endosome a GRK-phosphorylated GPCR can be dephosphorylated by a membrane associated phosphatase. This latter process allows for resensitization of the GPCR prior to it being recycled back to the plasma membrane where it can once again respond to ligand binding.
Table of Diseases/Disorders Associated with GPCR Defects
| Disease | Affected Receptor | Comments |
| Blomstrand chondrodysplasia | parathyroid hormone receptor 1, PTHR1 | manifests with remarkably advanced skeletal maturation at birth, loss-of-function mutation, autosomal recessive inheritance |
| central hypogonadism | gonadotropin releasing hormone receptor, GNRHR | impairment of pubertal maturation and reproductive function; loss-of-function mutation, autosomal recessive inheritance |
| central hypothyroidism | thyrotropin releasing hormone receptor, TRHR | characterized by insufficient TSH secretion resulting in low levels of thyroid hormones; loss-of-function mutation, autosomal recessive inheritance |
| color blindness | cone opsins | loss-of-function mutation, autosomal recessive inheritance, X-linked |
| congenital hypothyroidism | thyroid stimulating hormone receptor, TSHR | increased levels of plasma TSH and low levels of thyroid hormone; loss-of-function mutation, autosomal recessive inheritance |
| congenital night blindness | rhodopsin | congenital stationary night blindness (CSNB), loss-of-function mutation, autosomal dominant inheritance; numerous additional forms of CSNB are known and are characterized by impaired night vision, decreased visual acuity, nystagmus, myopia, and strabismus |
| familial ACTH resistance | adrenocorticotropic hormone ACTH | loss-of-function mutation, autosomal recessive inheritance |
| familial hypocalcemia | Ca2+ sensing receptor; CASR | characterized by hypocalcemia and hyperphosphatemia; can manifest with mild neuromuscular irritability, calcification of the basal ganglia, extrapyramidal disorders, cataracts, alopecia, abnormal dentition, coarse brittle hair, intellectual impairment, or personality disorders; gain-of-function mutation, autosomal dominant inheritance |
| familial hypocalciuric hypercalcemia | Ca2+ sensing receptor; CASR | hypercalcemic onset before age 10 years (unlike primary hyperparathyroidism), not accompanied by urinary stone or renal damage, pancreatitis and chondrocalcinosis are common, parathyroid hyperplasia is present in most patients and will persist after parathyroidectomy, loss-of-function mutation, autosomal dominant inheritance; the gain-of-function mutations in the CASR gene cause familial hypocalcemia |
| familial male precocious puberty | luteinizing hormone receptor, LHR | gain-of-function mutation, autosomal dominant inheritance |
| familial non-autoimmune hyperthyroidism | thyroid stimulating hormone receptor, TSHR | gain-of-function mutation, autosomal dominant inheritance |
| growth hormone deficiency | growth hormone releasing hormone receptor, GHRH | loss-of-function mutation, codominant inheritance |
| Hirschsprung disease susceptibility type 2 | endothelin receptor type B | classic Hirschsprung disease (type 1) is caused by defects in the RET gene which encodes a receptor tyrosine kinase receptor; type 2 Hirschsprung is also known as Waardenburg syndrome type 4A; loss-of-function mutation, complex mode of inheritance |
| ovarian dysgenesis 1 (ODG1), also called hypergonadotropic ovarian failure | follicle stimulating hormone receptor, FSHR | lack of menstruation accompanied by severe osteoporosis, gonadal dysgenesis, often with somatic abnormalities; loss-of-function mutation, autosomal recessive inheritance |
| Jansen metaphyseal chondrodysplasia | parathyroid hormone hormone receptor 1, PTHR1 | also known as metaphyseal dystosis, presents with extreme disorganization of the metaphyses of the long bones and of the metacarpal and metatarsal bones; gain-of-function mutation, autosomal dominant inheritance |
| male pseudohermaphroditism | luteinizing hormone/choriogonadotropin receptor, LHCGR | loss-of-function mutation, autosomal recessive inheritance |
| morbid obesity | melanocortin 4 receptor, MC4R | mutations in this gene are the most frequent genetic cause of severe obesity; MC4R binds α-melanocyte stimulating hormone (α-MSH); loss-of-function mutation, codominant inheritance |
| neonatal hyperparathyroidism | Ca2+ sensing receptor, CASR | manifests in the first 6 months of life with severe hypercalcemia, bone demineralization, and failure to thrive; loss-of-function mutation, autosomal recessive inheritance |
| nephrogenic diabetes insipidus | vasopressin V2 receptor, AVPR2 | symptoms include vomiting and anorexia, failure to thrive, fever, and constipation, caused by the inability of the renal collecting ducts to absorb water in response to antidiuretic hormone (ADH) which is also known as arginine vasopressin (AVP); loss-of-function mutation, X-linked inheritance |
| retinitis pigmentosa | rhodopsin | loss-of-function mutation, autosomal dominant and recessive modes of inheritance |
| sporadic hyperfunctional thyroid adenomas | thyroid stimulating hormone receptor, TSHR | gain-of-function mutation, somatic inheritance |
| sporadic Leydig cell tumors | luteinizing hormone/choriogonadotropin receptor, LHCGR | gain-of-function mutation, somatic inheritance |
