Last Updated: November 9, 2025
Gangliosides
Gangliosides are members of the glycosphingolipid family of glycolipids. Gangliosides are glycosphingolipids that are defined by the presence of N-acetylneuraminic acid (NANA; also known as sialic acid) in varying amounts. The specific names for gangliosides are a key to their structure. The first letter on the nomenclature, G, refers to ganglioside. The following letters, A, M, D, T, Q, and P, indicate that the molecule contains none (absent or asialo), mono-, di-, tri, quatra(tetra), or poly(more than 4)-sialic acid residues. The numerical designations, 1, 2 and 3, refer to the carbohydrate sequence that is attached to ceramide, see the Sphingolipid Metabolism and the Ceramides page for details.
Introduction to GM2 Activator Deficiency
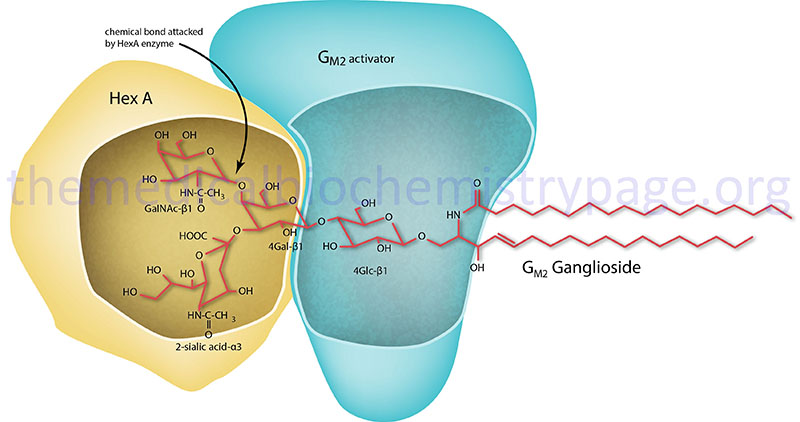
GM2 activator deficiency (also called Tay-Sachs AB variant) is an autosomal recessive disorder that is a member of the family of disorders identified as the GM2 gangliosidoses which are themselves members of the large family of diseases/disorders referred to as the lysosomal storage disorders. The GM2 gangliosidotic diseases are severe psycho-motor developmental disorders caused by the inability to properly degrade membrane associated gangliosides of the GM2 family (see Figure below). Because neural cell membranes are enriched in GM2 gangliosides, the inability to degrade this class of sphingolipid results in neural cell death. In addition to GM2 activator deficiency the family includes Sandhoff disease and Tay-Sachs disease. GM2 activator deficiency is an extremely rare disease with only a few cases identified world-wide.
Functions of GM2 Activator
GM2 ganglioside degradation requires the enzyme β-hexosaminidase and the GM2 activator protein (encoded by the GM2A gene). Hexosaminidase is a dimer composed of 2 subunits, either the α subunit (encoded by the HEXA gene) and/or the β subunit (encoded by the HEXB gene). The various isoforms of β-hexosaminidase result from the combination of α and β subunits. The HexS protein is a homodimer of αα, HexA is a heterodimer of αβ and HexB is a homodimer of ββ. It is the α-subunit that carries out the catalysis and only the HexA form of β-hexosaminidase can catalyze the cleavage of GM2 gangliosides (the bond cleaved is shown by the arrow in the Figure below). The activator first binds to GM2 gangliosides followed by hexosaminidase and then digestion occurs. The degradation of GM2 gangliosides takes place in the lysosomes. Failure to degrade these sphingolipids results in the lysosomes becoming engorged filling the cell, eventually choking off normal cellular functions. Because of the deposition of abnormal sphingolipids in the lysosomes the GM2 gangliosidotic diseases are also referred to as lysosomal storage disorders.
Molecular Biology of GM2 Activator
GM2 activator deficiency results from defects in the GM2A gene encoding the GM2 activator protein of the functional β-hexosaminidase enzyme complex. Deficiencies in the HEXA gene result in Tay-Sachs disease and deficiencies in the HEXB gene result in Sandhoff disease.
The GM2A gene resides on chromosome 5q33.1 spanning 16 kb and composed of 4 exons that generate two alternatively spliced mRNAs encoding a 193 amino acid precursor protein (isoform 1) and a 186 amino acid precursor protein (isoform 2). Mutations in the GM2A gene are very rare and only 7 pathogenic mutations have thus far (as of 2025) been characterized. All known forms of GM2 activator deficiency are of the infantile type.
Clinical Features of GM2 Activator Deficiency
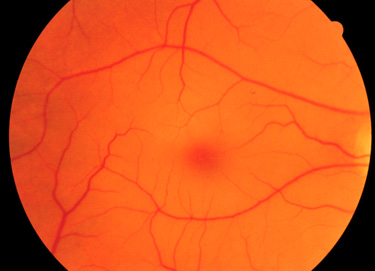
The clinical phenotypes associated with GM2 activator deficiency and the infantile forms of Tay-Sachs disease and Sandhoff disease are for the most part indistinguishable. Infants with GM2 activator deficiency appear normal at birth. Symptoms usually begin with mild motor weakness by 3 to 5 months of age. Parents will begin to notice that their afflicted child has a dull response to outside stimuli. Another early symptom is an exaggerated startle response (sudden extension of arms and legs) to sharp sounds. By 6 to 10 months of age infants will begin to show regression of prior acquired motor and mental skills. It is the loss of these activities that will normally prompt parents to seek a medical opinion. A progressive loss in visual attentiveness may lead to an ophthalmological consultation which will reveal macular pallor and the presence of the characteristic “cherry-red spot” on the fundus of the eye (see Figure below).
At around 8 to 10 months of age the symptoms of GM2 activator deficiency begins to progress rapidly. Infants are progressively non-responsive to parental stimulation. The exaggerated startle response becomes quite pronounced. Most frightening to parents is the onset of seizures which initially can be controlled by anti-seizure medication. However, the seizures become progressively more severe and are very frequent by the end of the first year. Psycho-motor deterioration increases by the second year and invariably leads to decerebrate posturing (typical of patients in persistent vegetative states), difficulty in swallowing and increased seizure activity. Ultimately, the patient will progress to an unresponsive vegetative state with death resulting from bronchopneumonia resulting from aspiration in conjunction with a depressed cough.
