Last Updated: October 28, 2025
Role of Protein C in Coagulation
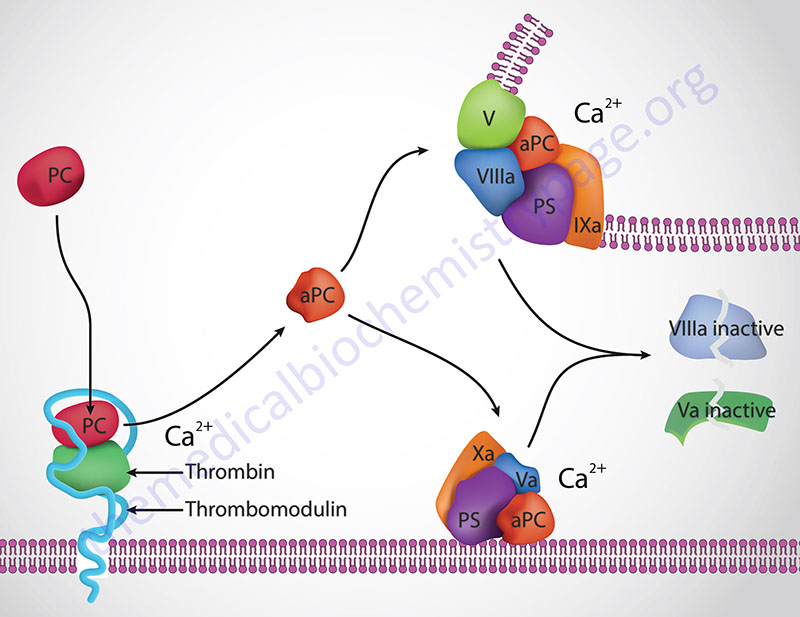
Protein C (PC) is a trypsin-like serine protease that serves as a major regulator of the coagulation process. Protein S (PS) serves as a co-factor for the functions of activated protein C (abbreviated aPC, and also APC).
Thrombin cleavage of PC removes the activation peptide generating aPC. When activated through cleavage by thrombin, aPC cleaves both activated factor V (designated Va) and activated factor VIII (designated VIIIa) into inactive enzymes. This results in the termination of the role of VIIIa as the scaffold for the formation of the tenase complex and Va as a co-factor in the conversion of prothrombin to thrombin in the prothrombinase complex. The cleavage of Va by aPC occurs at three Arg (R) residues, R306, R506, and R679.
The net effect, at the level of coagulation, of the activation of PC is termination of further increases in thrombin production and a halt to further fibrin clot formation. The activation of PC by thrombin occurs on the surface of the endothelium when thrombin binds to thrombomodulin and “captures” circulating PC. Following activation, aPC interacts with PS and then cleaves VIIIa and Va.
Molecular Biology of Protein C and Factor V
The protein C gene (PROC) is located on chromosome 2q14.3 and is composed of 8 exons that generate 12 alternatively spliced mRNAs that collectively encode 10 distinct preproprotein isoforms. Expression of the PROC gene is essentially exclusive to the liver with a 10-fold lower level of expression also found in the kidney
Protein C undergoes a series of post-translational modifications that include several N-linked glycosylation sites and γ-carboxylation of nine glutamate residues (gla residues) in the amino terminus. These gla residues in the amino terminus of PC constitute the “gla domain” of the protein. In addition to the gla domain, PC contains two epidermal growth factor-like regions (EGF domains), the serine protease domain, and an activation peptide.
The factor V gene (F5) is located on chromosome 1q24.2 and is composed of 25 exons that encode a 2224 amino acid preproprotein. Expression of the F5 gene is highest in the liver followed by the placenta. Factor V circulates in the blood in an inactive form until it is cleaved by thrombin. Thrombin cleavage of factor V yields a heavy and a light chain that are then held together in the presence of Ca2+ ions.
Factor V Leiden
The importance of aPC in controlling Va activity can be seen in the hypercoagulopathy (thrombophilia) referred to as factor V Leiden. This thrombophilia is caused by a mutation in the factor V gene resulting in a protein that is not effectively degraded by activated protein C (aPC). The factor V Leiden mutation is a single nucleotide (1691) change that generates a missense mutation of a glutamine (Q) at the R506 codon (designated R506Q) that is one of the aPC cleavage sites. Factor V Leiden is the most common inherited thrombophilia in Caucasian populations of European descent. Overall, 5% of the world population harbors the factor V Leiden mutation. The frequency of homozygosity for the R506Q mutation is around 1 in 5,000.
The symptoms of factor V Leiden are deep vein thrombosis (DVT) and pulmonary embolism, both of which can be fatal. In fact it is estimated that in as many as 30% of patients who experience an idiopathic or first event DVT and/or pulmonary embolisms are carriers of the factor V Leiden mutation. Loss of protein C results in massive and usually lethal thrombotic complications in infants with homozygous PC deficiency. In individuals who are heterozygous for PC deficiencies there is an increased risk for venous thrombosis.
