Last Updated: October 28, 2025
Introduction to von Willebrand Disease
von Willebrand disease (vWD) is caused by deficiencies in the protein von Willebrand factor (vWF). vWF is a multi-subunit glycoprotein that is found in subendothelial connective tissue, the blood, and in the α granules of platelets. vWD and vWF get their name from Erik von Willebrand who first described a bleeding disorder whose mode of inheritance was autosomal rather than X-linked (as in hemophilia A and hemophilia B). In addition, the disease resulted in bleeding from mucocutaneous sites (typical skin and mucous membranes) rather than from deep tissues and joints.
vWD is the most commonly occurring bleeding disorder with clinically significant forms affecting approximately 1 in 1000 individuals. However, abnormalities in vWF have been estimated to occur with a frequency of 1 in 100 persons, thus a relatively small fraction of persons harboring mutations in vWF are actually symptomatic.
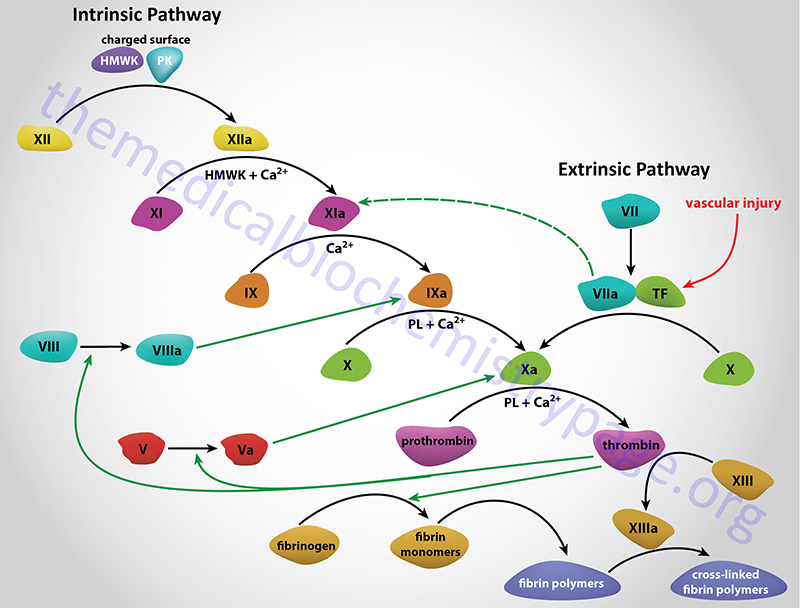
vWF is involved in two important reactions of hemostasis (blood coagulation). The first is the interaction of vWF with specific receptors on the surface of platelets and the subendothelial connective tissue. This interaction allows platelets to adhere to areas of vascular injury. The second role of vWF is to bind to and stabilize factor VIII allowing factor VIII to survive in the blood. As a consequence of these roles for vWF, deficiencies result in defective platelet adhesion and to secondary deficiencies in factor VIII. Since factor VIII deficiency results in hemophilia A, it is not surprising that vWF deficiencies can manifest with clinical symptoms that appear to be hemophilia A.
Molecular Biology of von Willebrand Disease
von Willebrand factor is encoded by the VWF gene. The VWF gene is located on chromosome 12p13.31 spanning approximately 180 kilobases (kb) and consists of 53 exons that encode a 2813 amino acid preproprotein. Numerous molecular alterations have been identified in vWD including nonsense and missense mutations and gene deletions.
Clinical Features of von Willebrand Disease
There are both dominant and recessive forms of vWD. The most common form of the disease (type 1) is an autosomal dominant disorder resulting from quantitative deficiency in vWF. Recessive inheritance of a virtual lack of vWF protein characterizes the clinically severe form of the disease called type 3 vWD. The inheritance of qualitative defects in vWF (dysfunctional protein) is characteristic of type 2 vWD. The type 2 category is further subdivided into four variants that are based upon the functional characteristics of the mutant protein. In type 2A vWD the residual vWF has reduced function. In type 2B vWD the mutant vWF has an increased affinity for the platelet surface protein GPIb (see the blood coagulation page for more information on the role of platelet glycoproteins in hemostasis). In type 2M vWD there is the presence of normal vWF multimeric complexes but they exhibit reduced platelet-dependent function. In type 2N vWD there are factor VIII deficiencies that are the result of mutations in vWF that do not bind to factor VIII.
In patients with type 1 and type 3 vWD (the quantitative deficiencies) the severity of the disease is related to the degree of functional deficiency in vWF. Clinically, type 1 and 3 vWD patients can manifest from insignificant problems to life threatening complications. Patients with type 2 vWD (the qualitative deficiencies) may exhibit symptoms whose severity may be greater than would be expected based upon a knowledge of the functional defect.
Symptoms of vWD often present shortly after birth. The common symptoms are easy bruising, bleeding from the gums, cutaneous hematomas and prolonged bleeding from cuts and abrasions. Individuals with severe forms of vWD can have bleeding complications because they either lack sufficient vWF to promote platelet adhesion at the site of vascular injury or they are factor VIII deficient due to the lack of the stabilizing action of vWF on factor VIII.
Treatment of von Willebrand Disease
The therapeutic response to vWD requires an understanding of the distinct functions of vWF. Mucocutaneous and post-surgical bleeding can be treated by raising the level of factor VIII. In order to address the platelet adhesion deficit in vWD it is necessary to increase the level of functional vWF. This can be accomplished using fresh frozen plasma or cryoprecipitates. The limitation to these approaches is that a large volume of frozen plasma is needed to deliver sufficient amount of vWF and cryoprecipitates are known to be associated with possible disease transmission. Platelet transfusions can be helpful in some patients with vWD, especially those who are refractory to factor VIII-vWF concentrates.