Last Updated: October 30, 2025
Introduction to Variegate Porphyria
Variegate porphyria (VP) is an autosomal dominant disorder that is a member of a family of disorders referred to as the porphyrias. VP is also known by the names porphyria variegata, protocoproporphyria and South African genetic porphyria. The disease is quite common in South African whites (hence the related name of this disorder) with a frequency of 3 cases per 1000 persons. This high incidence has been traced to a founder couple who emigrated from Holland and were married in 1688.
Each of the porphyrias results from deficiencies in a specific enzyme involved in the biosynthesis of heme (also called the porphyrin pathway). The term porphyria is derived from the Greek term porphura which means “purple pigment” in reference to the coloration of body fluids in patients suffering from the originally described porphyria which is now known as porphyria cutanea tarda (PCT).
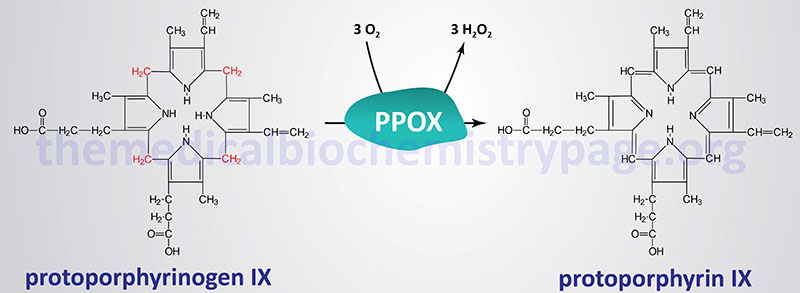
The porphyrias are classified on the basis of the tissue that is the predominant site of accumulation of metabolic intermediates. These classifications are “hepatic” or “erythroid”. Each disease is also further characterized as being acute or cutaneous dependent upon the major clinical features of the disease. VP is classified as an acute hepatic porphyria. VP results from defects in protoporphyrinogen oxidase.
Molecular Biology of Variegate Porphyria
The protoporphyrinogen oxidase gene (PPOX) is located on chromosome 1q23.3 spanning 8 kb and encompassing 18 exons that generate six alternatively spliced mRNAs, that collectively encode four protein isoforms.
Several different mutations have been identified in the PPOX gene resulting in VP. These mutations include missense, nonsense, and splice-site mutations as well as deletions and insertions. Except for the mutation found in the ancestors of the South African founder couple, most mutations in the PPOX gene are unique to each affected patient. The South African founder mutation is a missense mutation where tryptophan is substituted for arginine at amino acid 59 (R59W).
Clinical Features of Variegate Porphyria
The clinical manifestations of VP are highly similar to other acute porphyrias such as acute intermittent porphyria, AIP. The term “variegate” assigned to this particular porphyria relates to the fact that the disease can present with neurological manifestations, cutaneous photosensitivity or both. Common symptoms include cutaneous photosensitivity, abdominal pain, constipation, nausea, vomiting, hypertension, tachycardia, neuropathy and back pain. Disorientation and frank psychosis may be conspicuous features of VP.
The symptoms of VP are rarely manifest prior to puberty. However, individuals homozygous for PPOX mutations exhibit severe clinical manifestations of VP at the outset of childhood. Clinical manifestation in VP becomes apparent in the event of an increased demand for hepatic heme production. This most often occurs due to drug exposure or some other precipitating factor such as an infection similar to the precipitating factors in AIP. If a drug is the cause of an attack its use should be discontinued immediately. Women using oral contraceptives are particularly susceptible to the cutaneous manifestations of VP. Most individuals (at least 75%) who inherit a PPOX mutation are asymptomatic throughout life.
Treatment of Variegate Porphyria
Treatment of VP involves a high carbohydrate diet and during a severe attack an infusion of 10% glucose is highly recommended, which is the same recommended intervention in treating acute attacks in the related porphyria, acute intermittent porphyria, AIP.
The metabolic and molecular logic behind the use of glucose infusion in acute porphyric attacks stems from the fact that hypoglycemia induces the expression of the transcriptional co-activator PGC-1α (peroxisome proliferator activated receptor-γ co-activator 1α). PCG-1α is a major transcriptional regulator of hepatic genes involved in gluconeogenesis and is also an activator of the ALAS1 gene. Thus, hypoglycemia can precipitate acute attacks in VP patients (as well as other porphyrias) due to increased synthesis of ALAS1. Hemin arginate is used to treat acute attacks of variegate porphyria but is not effective in treating the cutaneous symptoms associated with the disorder.
