Last Updated: October 28, 2025
Introduction to Sickle Cell Anemia
Sickle cell anemia, also called sickle cell disease (SCD), is an autosomal recessive disorder affecting the function of hemoglobin. In order for full disease symptoms to manifest in an individual they must carry two copies (homozygous genotype = SS or HbSS) of the HbS gene. However, individuals who are heterozygous (genotype = AS or HbAS) have what is referred to as sickle cell trait, a phenotypically dominant trait. Although AS individuals are clinically normal their red blood cells can sickle under very low oxygen pressure, e.g. when at high altitudes in airplanes with reduced cabin pressure. Because of this phenomenon, AS individuals exhibit phenotypic dominance yet are recessive genotypically.
There are several genetic conditions besides the SS genotype that can result in red blood cell sickling. One condition is termed sickle beta 0 (Sβ0) thalassemia which is the result of compound heterozygosity where one β-globin allele is mutated such that no protein is made. Sickle cell disease type SC results from compound heterozygosity of the HbS allele with the HbC allele. The HbC allele results when codon 6 is mutated (GAG to AAG) to a lysine codon, designated, G6K. Although other compound heterozygosities have been identified involving the adult β-globin gene, they are quite rare in comparison to HbSS, Sβ0, and HbSC.
Genetics of Sickle Cell Anemia
Mutations in the globin genes that alter the protein composition but not necessarily the amount of expression are referred to as qualitative mutations. Of the mutations leading to qualitative alterations in hemoglobin, the missense mutation in the β-globin gene that causes sickle cell anemia is the most common.
The mutation causing sickle cell anemia is a single nucleotide substitution (A to T) in the codon for amino acid 6. The change converts a glutamic acid codon (GAG) to a valine codon (GTG). This mutation is designated G6V. The form of hemoglobin in persons with sickle cell anemia is referred to as HbS.
The nomenclature for normal adult hemoglobin protein is HbA1. Adult red blood cells also carry another minor form of adult hemoglobin (about 2% of the total) identified as HbA2. The HbA1 heterotetramer is composed of two α-globin peptides and two β-globin peptides. The HbA2 heterotetramer is composed of two α-globin peptides and two δ-globin peptides.
The human β-globin gene cluster contains several genes whose 5′ to 3′ orientation on chromosome 11 reflects the ontogeny of their expression from embryonic globin [episilon (ε) gene], to fetal β-globin [gammaG (γG) and γA genes], to adult β-globin [weakly the delta (δ) gene followed by the β gene]. The β-globin gene (HBB) is located at 11p15.5 and is composed of 3 exons that encode a 147 amino acid protein.
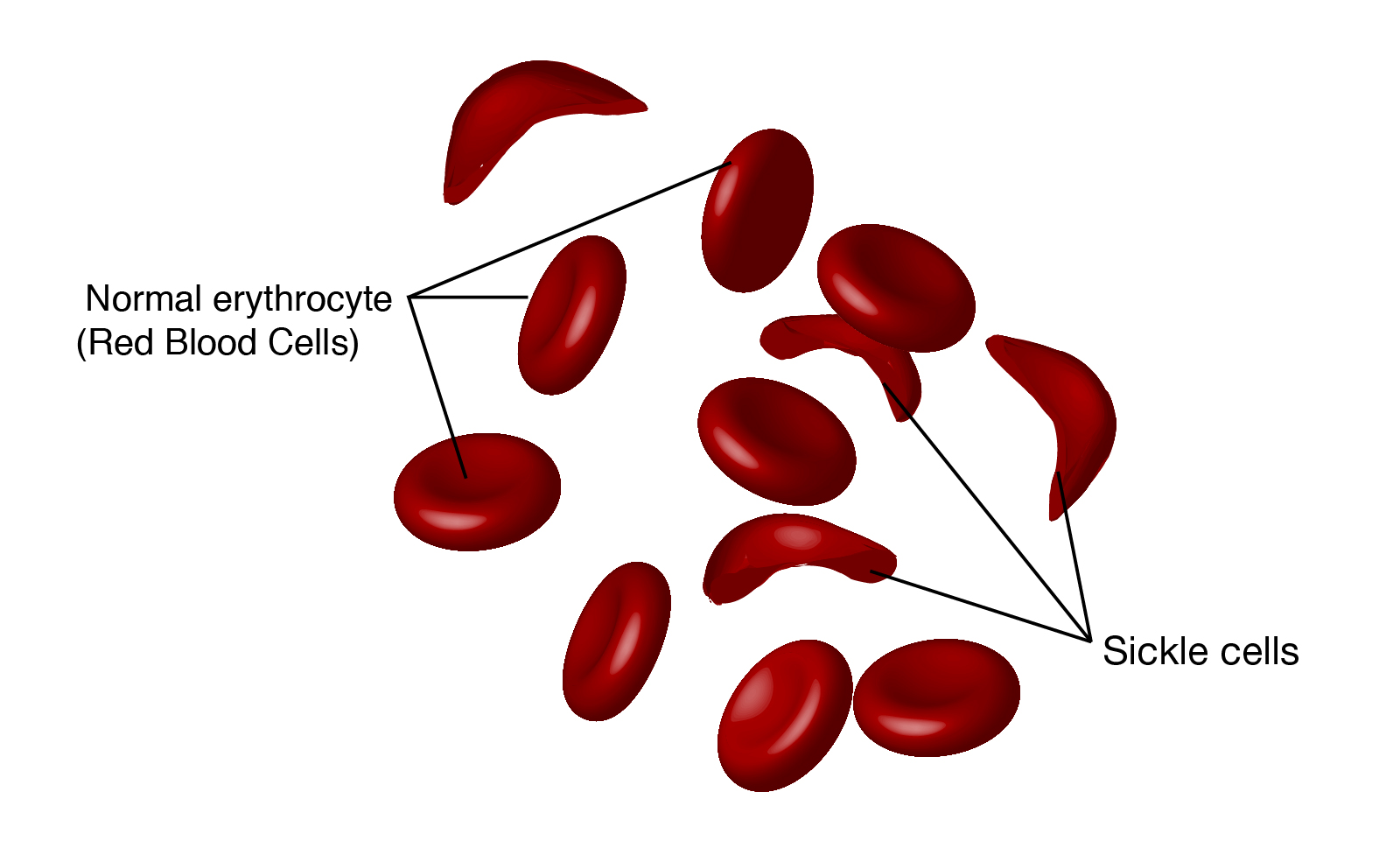
The underlying problem in sickle cell anemia is that the valine for glutamic acid substitution results in hemoglobin tetramers that aggregate into arrays upon deoxygenation in the tissues. This aggregation leads to deformation of the red blood cell into a sickle-like shape making it relatively inflexible and unable to traverse the capillary beds. This structural alteration in the red blood cell can easily be seen under light microscopy and is the source of the name of this disease. Repeated cycles of oxygenation and deoxygenation lead to irreversible sickling.
Pathophysiology of Sickle Cell Anemia
Sickle cell anemia is characterized by persistent episodes of hemolytic anemia and the occurrence of acute episodes referred to as sickling crises. The sickling red cells result in clogging of the fine capillary beds. In addition, due to these recurrent vasculo-occlusive episodes there are a series of complications. Because bones are particularly affected by the reduced blood flow, frequent and severe bone pain results. This is the typical symptom during a sickle cell crisis. Long term, the recurrent clogging of the capillary beds leads to damage to the internal organs, in particular the kidneys, heart, and lungs. The continual destruction of the sickled red blood cells leads to chronic anemia and episodes of hyperbilirubinemia.
Sickle cell anemia usually presents in infancy although milder cases will only manifest later in life. The hallmark presentations are failure to thrive and repeated infections and repeated attacks of painful hand-foot syndrome, termed dactylitis. This symptom is characterized by sudden onset of a painful swelling of the back (dorsum) of the hands and feet. Presenting infants will be pale with slight jaundice (icterus) visible in the sclera of the eyes and the spleen will be palpable. The clinical course of sickle cell anemia is variable even within the afflicted individuals of the same sibship.
Children with sickle cell anemia have an increased susceptibility to infections due to Streptococcus pneumonia, Salmonella, and Hemophilus influenza. The increased propensity to infection results in impaired splenic function that resembles infections in children who have had their spleens removed for other reasons. Pneumococcal pneumonia and septicemia are the most devastating consequences of sickle cell anemia. Septicemia and shock is a common cause of death particularly in infants and during childhood.
Laboratory Values Associated with Sickle Cell Anemia
| Lab Value | Normal | Sickle Cell Anemia |
| MCV (fL) | 82–98 | 88.1 ± 6.8 |
| MCH (pg) | 26–34 | 29.8 ± 2.4 |
| Hemoglobin (g/dL) | 12–16.5 | 6–11 |
| HbA1 (%) | 95 | 0 |
| HbA2 (%) | 2–3 | 3.5–5.7 |
| HbF (%) | 0.5–2 | 2.9–7.7 |
| HbS (%) | 0 | >90 |
Treating Sickle Cell Anemia
The only cure for sickle cell anemia is a bone marrow transplant. However, this treatment can be highly risky in the already compromised condition of sickle cell anemia patients.
Treatments that are used to alleviate symptoms include antibiotics (predominantly penicillin) to prevent infections, particularly in the lungs, pain medications to lessen the pain associated with the frequent vaso-occlusive crises, medications to prevent red blood cell sickling, and blood transfusions.
The use of hydroxyurea to increase the expression of the fetal gene of the beta globin cluster (HbF) has been shown to provide relief in some sickle cell anemia patients as well as in compound heterozygotes with one S allele and one β-globin mutation. These latter patients are identified as Sβ thalassemia individuals.
Voxelotor (trade name Oxbryta®) is used to prevent red blood cell sickling in sickle cell disease. The drug is orally administered to adults and children 4-years-old and older. By preventing sickling voxelotor reduces the destruction of some red blood cells, which in turn, improves overall blood flow and also may lower the risk for developing anemia. The use of voxelotor is not without side-effects that include a risk for nausea, diarrhea, abdominal pain, headaches, fatigue, and fever.
The monoclonal antibody, crizanlizumab-tmca (trade name Adaveko®) has been approved for use in adults and children 16-years-old and older who have sickle cell disease as a means to reduce vaso-occlusive crises and the associated pain. The monoclonal antibody binds to the cell adhesion molecule, P-selectin, on the surface of activated platelets and endothelial cells which prevents interactions between red blood cells, endothelial cells, platelets, and leukocytes. The reduction in red blood cell interaction with the endothelium reduces the likelihood for vessel blockage, inflammation, and the resultant pain crises.
In addition to more classical medications to treat mild to moderate pain such as acetaminophen or ibuprofen, severe pain can be treated in a hospital setting with the use of opiates. The use of L-glutamine in sickle cell disease patients age 5 and older has been shown to lower the number of pain crises.
Sickle Cell Trait
Individuals that are heterozygous with one normal adult β-globin (HbA) gene and one sickle cell β-globin gene (HbS), genotype HbAS (or just AS), have what is referred to as sickle cell trait. Whereas individuals with sickle cell anemia (HbSS) will have no detectable HbA1 in their blood, because sickle cell trait individuals have one normal adult β-globin gene their levels of HbA1 constitute 55%–60% of the total globin proteins while the level of HbS is 35%–40% of the total.
Unlike the situation in individuals with sickle cell anemia, those with sickle cell trait do not often experience episodes of vaso-occlusive crisis. However, patients with sickle cell trait may experience symptoms the same as in sickle cell anemia if exposed to conditions that favor sickling. These conditions include severe hypoxia, dehydration, increased sympathetic outflow, hypothermia, hyperthermia, high 2,3-BPG levels, or enhanced inflammatory status.
Under conditions of hypoxia the red blood cells of sickle cell trait individuals will deform to the typical sickle shape of sickle cell anemia patients. The sickled cells will adhere to vascular walls resulting in blockage of blood vessels in the muscles, kidneys, and other organs, resulting in tissue necrosis. Other cells, particularly immune cells and platelets, interact with the sickled red blood cells causing increased adhesion. This phenomenon occurs in multiple organs in the body, including the heart, lungs, abdomen, and kidneys. As a result of repeated episodes of hypoxia, or other causes of red blood cell sickling, in individuals with sickle cells trait, the constant ischemia can lead to organ damage just as in the case of sickle cell anemia.
Despite the fact that patients with sickle cell trait can experience many complications like papillary necrosis, asymptomatic bacteriuria, splenic infarction, and exercise-induced death, the prognosis of patients with sickle cell trait is promising. Indeed, the average life expectancy of people with the sickle cell trait is the same as that of the general population. On the other hand, sickle cell disease is associated with a significantly increased risk of in-hospital mortality, whereas sickle trait does not.