Last Updated: October 31, 2025
Introduction to Menkes Disease
Menkes disease is inherited as an X-linked recessive disorder of copper homeostasis. The disorder is associated with an inability to absorb copper from the gastrointestinal tract and an inability of tissues to absorb copper from the blood. This results in the reduced, or loss of, function of copper-dependent proteins. In cells copper, like, iron, can exist in the cuprous (Cu+) and cupric (Cu2+) states. Copper-dependent proteins that carry out red-ox reactions, such as lysyl oxidase and cytochrome c oxidase have their copper alternating between these two states during the course of their catalyzed reactions.
As a consequence of the reduced delivery of copper to the brain, Menkes patients exhibit severe mental and developmental impairment. In addition, defective copper-dependent protein function within the brain contributes to the severe neural degeneration, primarily due to iron overload as a consequence of loss of ceruloplasmin function (see Clinical Features below).
Menkes patients also exhibit connective tissue abnormalities and twisting of blood vessels where there are normally supposed to be turns. The vessel twisting can lead to blockages if it is severe. The loss of functional connective tissue is due to a loss of the activity of the extracellular copper-dependent collagen processing enzyme, lysyl oxidase. Due to the critical role of lysyl oxidase in the generation of functional collagen, the resultant abnormal collagen leads to impaired platelet adhesion at sites of vessel injury with consequent increased bleeding times. Menkes disease is so named because it was originally described in 1962 by Dr. John H. Menkes. The incidence of Menkes disease ranges from 1:40,000 to 1:360,000 live births.
Molecular Biology of Menkes Disease
Menkes disease is the result of defects in the P-type ATPase protein that is responsible for the translocation of copper across the intestinal basolateral membrane into the blood, thus allowing for uptake of dietary copper. This protein is encoded by the ATPase, Cu2+-transporting, alpha polypeptide (ATP7A) gene.
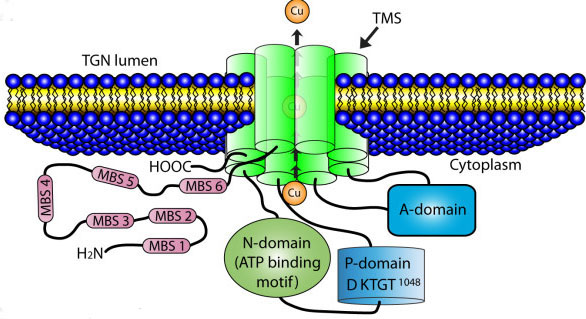
The ATP7A gene is located on the X-chromosome (Xq21.1) and is composed of 23 exons that generate two alternatively spliced mRNAs encoding isoform 1 (1500 amino acids) and isoform 2 (1422 amino acids).
The ATP7A protein contains an ATPase domain, a hinge domain, a phosphorylation site, and six copper-binding sites. The structure of the ATP7A gene is highly similar (57% identity at the amino acid level) to that of the ATP7B gene which is disrupted in another copper-transport defect disease called Wilson disease. ATP7A has two important activities related to copper homeostasis. It delivers copper to several copper-requiring enzymes and is required for the ATP-driven efflux of copper from cells. ATP7A is expressed in numerous tissues except the liver. Copper homeostasis effected by the liver is the function of the ATP7B protein.
When carrying out its function to supply copper-dependent enzymes with copper, the ATP7A protein is localized to the trans-Golgi network where it transports copper into the lumen of the Golgi. When copper levels rise, ATP7A is translocated to vesicular compartments closely associated with the plasma membrane where it can transport copper into these compartments. The copper in these vesicles can then be released by exocytosis.
Numerous mutations have been identified in the ATP7A gene resulting in Menkes disease. To date at least 357 different mutations have been characterized. The most frequently occurring mutations, accounting for 22% of all cases, are insertion or deletion of a few base pairs. Additional alterations include missense mutations, partial gene deletions, splice site mutations, and mutations leading to premature termination of translation.
Clinical Features of Menkes Disease
The clinical spectrum associated with Menkes disease is due in large part to the reduced activity of cytochrome c oxidase, dopamine β-hydroxylase, and ceruloplasmin, all of which are critical Cu2+-dependent enzymes. The clinical spectrum of Menkes disease includes progressive neurodegeneration, connective tissue abnormalities and wiry brittle hair. The distinctive clinical features of Menkes disease are usually present by 3 months of age. Infants will lose, or fail to demonstrate, specific developmental milestones and failure to thrive. Most patients with Menkes disease do not survive beyond early childhood, however there is phenotypic variability and some mildly affected patients have been reported to survive for longer periods. Because of the phenotypic variations, Menkes disease has been divided into three classifications. Classic Menkes disease which results in early death, mild Menkes disease with longer survival times (accounting for 6% of all patients), and occipital horn syndrome, OHS (accounting for 3% of all patients). OHS was previously called X-linked cutis laxa, or Ehlers-Danlos syndrome type IX.
In the classic form of Menkes disease infants have cherubic faces, sagging jowls, and no or scant eyebrows. Their hair is gray or white due to the lack of tyrosinase activity and has the appearance and feel of steel wool. The deficiency in lysyl oxidase activity accounts for most of the skeletal abnormalities present in Menkes infants. These anomalies include osteoporosis, metaphyseal dysplasia, wormian skull bones, and rib fractures. Defective collagen processing, in addition to resulting in connective tissue and skeletal anomalies, also results in impaired platelet adhesion to exposed sub-endothelial extracellular matrices. The loss of effective platelet adhesion leads to impaired platelet activation at sites of vessel injury which in turn leads to dramatically prolonged bleeding times in Menkes patients.
Progressive cerebral degeneration in Menkes disease occurs primarily due to loss of the Cu2+-dependent enzymes, ceruloplasmin, cytochrome c oxidase, peptidylglycine α-amidating monooxygenase, and dopamine β-hydroxylase in the central nervous system and to the loss of iron homeostasis in the brain. The defective brain iron homeostasis is due to the loss of activity of the ferroxidase, ceruloplasmin. Within most tissues, ceruloplasmin is bound to the plasma membrane via a GPI linkage and the function of GPI-ceruloplasmin is iron efflux from the tissues. Without adequate iron homeostasis, the brain accumulates iron resulting in the toxic effects of iron overload. The iron overload due to defective, or no, ceruloplasmin activity is a form of hemochromatosis termed hemosiderosis.
Unfortunately there is no effective treatment for Menkes disease and severely afflicted infants will not survive more than a few months after birth. In mildly afflicted patients there is some benefit to parenteral administration of various forms of copper such as copper histidine, copper chloride, and copper sulfate. Although this treatment can correct the hepatic copper deficiency, normalize serum copper, and ceruloplasmin levels it has not been shown to ameliorate the progressive neurologic deterioration.