Last Updated: October 28, 2025
Introduction to Hemophilia A
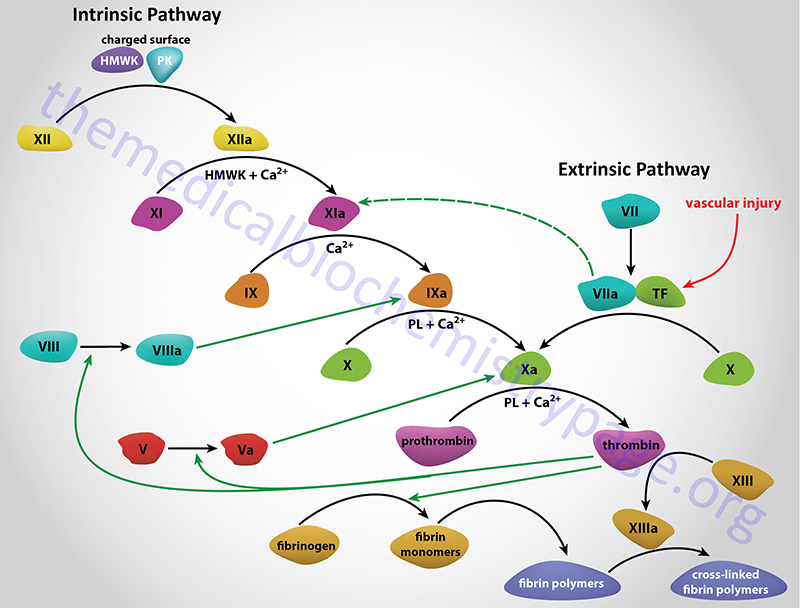
Hemophilia A is referred to as classic hemophilia and was first recognized in the second century AD. The disease is an X-linked recessive bleeding disorder caused by defects in the clotting cascade enzyme factor VIII. Factor VIII serves as a cofactor in the activation of factor X to Xa in a reaction referred to as the “tenase” complex. The actual enzyme responsible for activating factor X to Xa is activated factor IX (factor IXa). Deficiencies in factor IX result in the related bleeding disorder, hemophilia B.
Molecular Biology of Hemophilia A
Factor VIII is encoded by the F8 gene. The F8 gene resides near the telomeric end of the long arm of the X chromosome (Xq28). The gene spans 186 kilobases (kb) and is composed of 27 exons that generate two alternatively spliced mRNAs. These two mRNAs encode factor VIII isoform a (2351 amino acids) and isoform b (216 amino acids).
The fully mature factor VIII protein contains 2,332 amino acids. The smaller isoform b contains the phospholipid binding domain of the larger factor VIII protein. The mRNA that encodes factor VIII isoform b contains a 5′ exon that resides within intron 22. This 5′ exon, that codes for eight amino acids, is spliced to exons 23–26.
A total of 2,537 mutations have been identified in the F8 gene leading to hemophilia A. These mutations include frameshift mutations, missense mutations, nonsense mutations, gene inversions, large deletions, and splicing errors.
Clinical Features of Hemophilia A
Patients with hemophilia A suffer from joint and muscle hemorrhage, easy bruising and prolonged bleeding time from wounds. Almost all patients with hemophilia A have normal platelet function so the bleeding from minor cuts or abrasions is generally not severe. Hemophilia A can be divided into severe or moderate disease. Approximately 50%–60% of hemophilia A patients suffer from the severe form of the disease and have <1% normal factor VIII activity. Patients with moderate hemophilia A have from 1%–20% of normal factor VIII.
Because hemophilia A is an X-linked disease almost all patients are male and the occurrence world-wide is 1 in 5,000 male births. It is possible that an affected female can result from the mating of an affected male with a carrier female but the Mendelian frequency for such a situation is approximately 1 in 50 million female births. Nonetheless, there have been cases described of female hemophilia A patients. A more common mechanism for presentation of hemophilia A in females is the process of X chromosome inactivation which could lead to inactivation of the X chromosome that harbors the wild-type factor VIII gene. Other rare possibilities also exist for the generation of female hemophilia A but are not discussed here.
The frequency and severity of the bleeding in hemophilia A patients is inversely correlated to the level of residual factor VIII protein circulating in the blood. The weight bearing joints are the ones most affected in the disease and include the hips, knees, ankles and elbows. If the bleeding in the joints is left untreated it will lead to severe swelling and pain, joint stiffness and inflammation. Blood in the synovial fluid of the joints is highly irritating causing synovial overgrowth and a tendency to cause additional bleeding from the vascular tissues of the joint. The bleeding results in the deposition of iron in chondrocytes with the consequences being the development of degenerative arthritis. Muscle bleeding, like joint bleeding, is most prevalent in large load-bearing muscle groups such as in the thigh, calf, buttocks and posterior abdominal wall. Since all of these untoward clinical symptoms are the result of a lack of factor VIII in the blood, replacement of factor VIII by intravenous infusion completely normalizes the hemophiliac’s blood coagulation (hemostasis) processes.
Treatment of Hemophilia A
The treatment of patients with hemophilia A primarily involves replacing the missing factor VIII clotting factor. Current therapy involves the use of recombinant human factor VIII as the primary treatment protocol. The use of human factor VIII derived from plasma is still being employed but is only considered a secondary line of therapy. The limitation of the use of recombinant humans factor VIII is the short half-life in the blood (~12 hr) compared to naturally derived coagulation factors. The conjugation of recombinant factor VIII to either IgG1, albumin, or polyethylene glycol (PEG) have lead to longer half-lives of recombinant factor VIII.