Last Updated: October 28, 2025
Introduction to Factor XIII
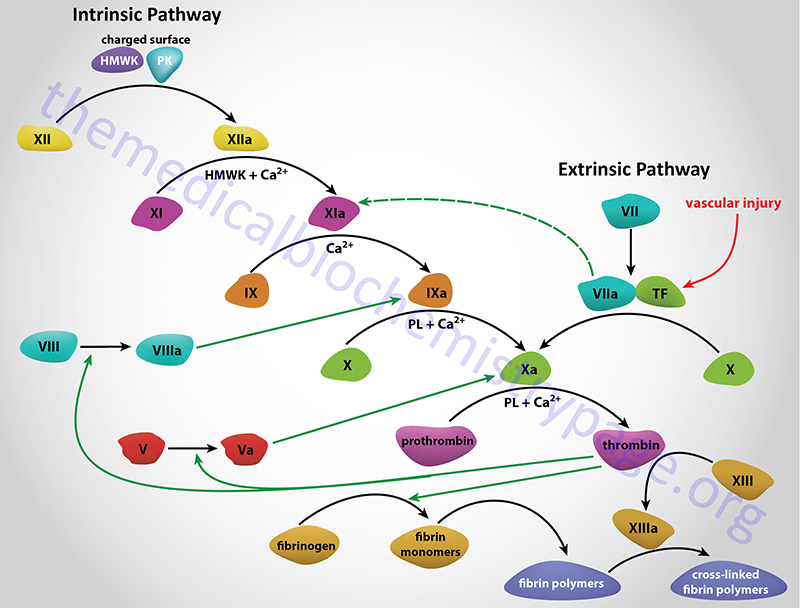
Factor XIII is also called protransglutaminase, fibrin stabilizing factor (FSF), and fibrinoligase. Factor XIII is found both in the plasma and in platelets. The plasma version is a heterodimeric enzyme composed of two A and two B subunits. The A subunit possesses the catalytic activity. The platelet version of factor XIII is composed of the A subunits only.
Factor XIII is activated (XIIIa) by thrombin in presence of Ca2+. Following activation of plasma factor XIII the B subunits are dissociated resulting in the same enzyme as in platelets. The function of XIIIa is to cross-link fibrin monomers thereby stabilizing the fibrin clot.
Deficiency of factor XIII is inherited as an autosomal recessive trait and can be due to mutations in both the genes encoding the A and B subunits (type I factor XIII deficiency) or just the gene encoding the A subunit (type II factor XIII deficiency).
Molecular Biology of Factor XIII
The A subunit is encoded by the F13A1 gene. The F13A1 gene is located on chromosome 6p25.1 and is composed of 15 exons encoding a 732 amino acid preproprotein.
The B subunit is encoded by the F13B gene. The F13B gene is located on chromosome 1q31.3 and is composed of 13 exons that encode a 661 amino acid preproprotein.
Clinical Features of Factor XIII Deficiency
Deficiency in factor XIII is characterized by delayed bleeding even though primary hemostasis is normal. Typical symptoms include neonatal bleeding from the umbilical cord, intracranial hemorrhage, soft tissue hematomas, recurrent spontaneous miscarriage, and abnormal wound healing. Umbilical cord bleeding after birth occurs in over 90% of afflicted individuals. Intracranial bleeding occurs in about 25% of individuals and is the leading cause of mortality in factor XIII deficiency.
Factor XIII, of the blood coagulation process, is also known as fibrin-stabilizing factor or fibrinoligase. Factor XIII is a heterotetramer composed of two A chains and two B chains (A2B2) held together by non-covalent bonds. The active site of the complex is within the A subunits and the B subunits are thought to stabilize the A subunits or serve as plasma carrier subunits. Thrombin cleaves a small peptide from the A chains which activates the transamidase activity.
Factor XIII is converted to its active form (XIIIa) by thrombin in the presence of Ca2+. The reaction catalyzed by factor XIIIa is the formation of γ-glutamyl-ε-lysine bonds between fibrin monomers as well as between fibrin and α2-plasmin inhibitor. Other proteins such as fibronectin, actin, and myosin have been shown to be substrates for factor XIIIa-induced cross-linking.
Patients who lack both the A and B subunits of factor XIII exhibit what is referred to as type I factor XIII deficiency. Most patients with factor XIII deficiency only lack functional subunit A protein with a frequency of around 1 in 5 million individuals. This form of the disorder is referred to as type II factor XIII deficiency. Factor XIII deficiency is normally treated with fresh frozen plasma, cryoprecipitate, or crude factor XIII concentrate from placenta.