Last Updated: October 28, 2025
Introduction to Factor XI
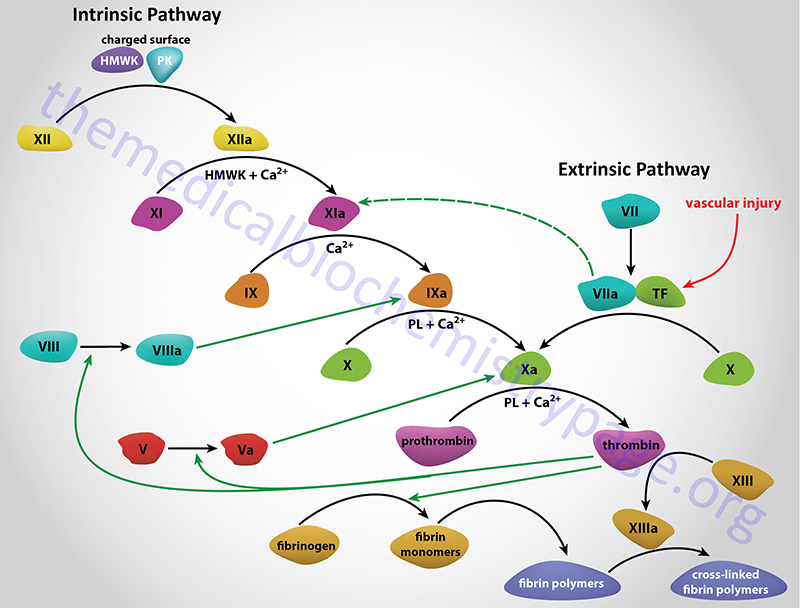
Factor XI is historically known as plasma thromboplastin antecedent (PTA). Factor XI participates in the intrinsic coagulation cascade. Factor XI is activated to XIa by a complex of high molecular weight kininogen (HMWK), activated factor XII (XIIa), and Ca2+. Activated factor XI in turn activates factor IX to IXa in the presence of phospholipids and Ca2+.
Factor XI can function as a bridge between the intrinsic and extrinsic coagulation cascades given that factor VII, which is activated by tissue factor (TF) released at the sites of vascular injury, can participate in the activation of factor XI. In addition, the factor IXa, generated by the action of factor XIa, can activate factor VII leading to an amplification of interactions between the extrinsic and intrinsic pathways.
Molecular Biology of Factor XI
Factor XI is encoded by the F11 gene. The F11 gene is located on chromosome 4q35.2 and is composed of 16 exons that generate two alternatively spliced mRNAs. These mRNAs encode preproproteins of 625 amino acid (isoform 1) and 162 amino acids (isoform 3).
The mature isoform 1 protein circulates in the blood as a disulfide bonded homodimer. Activated factor XII (XIIa) cleaves both subunits in the circulating homodimer generating two heavy and two light chains that remain held together by the disulfide bonds. The heavy chain of comprises 369 amino acids and the light chain comprises 238 amino acids. The light chain contains the catalytic domain of factor XI which is similar to the catalytic domain in other members of the trypsin family of serine proteases.
At least twenty distinct mutations (missense mutations and deletions) have been identified in patients suffering from factor XI deficiency all of which are associated with low levels of factor XI and low activity. Most of the mutations, as well as afflicted individuals, are of Jewish ancestry with the first patients identified being of Ashkenazi Jewish descent. The frequency of this disorder in Ashkenazi Jewish individuals in as high as 1 in 190.
Clinical Features of Factor XI Deficiency
A deficiency in the blood coagulation factor XI (also called plasma thromboplastin antecedent, PTA, deficiency) was originally identified as hemophilia C by Rosenthal and coworkers in 1953. Deficiency in factor XI is inherited as an autosomal recessive disease with clinical manifestations seen in both homozygotes and heterozygotes. Bleeding tendency in factor XI deficient patients is less severe and much more variable than in hemophila A or hemophilia B.
Bleeding in factor XI deficiency is most often triggered by surgery or trauma. Spontaneous bleeding, as occurs in both hemophilia A and B, is very rare in factor XI deficiency. Excessive bleeding from sites of injury can persist for several hours to days. However, there is a variability to the severity of bleeding where some individuals with levels of factor XI that are <1% of normal do not bleed excessively despite trauma. This phenotypic phenomenon is the result of both the inherited genotype as well as the site of injury. Bleeding due to surgery or trauma on tissues with a high fibrinolytic property, such as the oral cavity, is the most severe.