Last Updated: January 2, 2024
Introduction to the β-Thalassemias
The human β-globin gene cluster is located on chromosome 11 (specifically 11p15.4) and includes the genes that encode the epsilon globin (ε: embryonic), the gamma A and gamma G globins (Aγ; Gγ: fetal), the delta globin (δ: adult), and the beta globin (β: adult).
A large number of mutations have been identified leading to decreased or absent production of β-globin chains resulting in the β-thalassemias. The majority of β-thalassemias are inherited with an autosomal recessive pattern. In the most severe situation mutations in both the maternal and paternal β-globin genes leads to loss of normal amounts of β-globin protein.
There are forms of β-thalassemia identified as the dominantly inherited β-thalassemias or “inclusion body β-thalassemias”. These forms of β-thalassemia are heterogeneous at the molecular level due to mutations at or near the HBB gene. Many of the mutations associated with inclusion body β-thalassemia reside in exon3 of the HBB gene. These mutations include frameshifts, nonsense mutations, and complex rearrangements that result in the synthesis of truncated or elongated and volatile β-globin proteins that are very unstable.
At the level of the β-globin proteins the β-thalassemias are characterized by whether or not there is partial or complete defect in protein production. A complete lack of protein is denoted as β0-thalassemia (beta-zero-thalassemia). If mutation(s) allows production of a small amount of functional β-globin then the disorder is denoted as β+-thalassemia (beta-plus-thalassemia). Clinically the β-thalassemias can be divided into three categories:
Classification of β-Thalassemias
Thalassemia Major
Thalassemia major patients require frequent blood transfusions for survival. Both β0– and β+-thalassemias are referred to as thalassemia major, also called Cooley anemia after Dr. Thomas Cooley who first described the disorder.
Thalassemia Intermedia
Thalassemia intermedia patients exhibit intermediate levels of severity. The term thalassemia intermedia is used to designate individuals with significant anemia and who are symptomatic but unlike thalassemia major do not require transfusions. This syndrome results in individuals where both β-globin genes express reduced amounts of protein or where one gene makes none and the other makes a mildly reduced amount. A person who is a compound heterozygote with α-thalassemia and β+-thalassemia will also manifest as thalassemia intermedia.
Thalassemia Minor
Thalassemia minor patients are heterozygous for β-thalassemia. Afflicted individuals harbor one normal β-globin gene and one that harbors a mutation leading to production of reduced or no β-globin. Individuals that do not make any functional β-globin protein from 1 gene are termed β0 heterozygotes. If β-globin production is reduced at one locus the individuals are termed β+ heterozygotes. Thalassemia minor individuals are generally asymptomatic.
The primary cause of the α-thalassemias is deletion, whereas, for β-thalassemias the mutations are more subtle. Over 170 different mutations have been identified resulting in the β-thalassemias. These mutations include gene deletions, point mutations in the promoter, mutations in the coding region leading to defective initiation, insertions and deletions resulting in frameshifts and nonsense mutations, point mutations in the polyadenylation signal, and an array of mutations leading to splicing abnormalities.
Molecular Biology of β-Thalassemias
All of the beta globin cluster genes are located at 11p15.4 and are composed of 3 exons and encode proteins of 147 amino acids. The embryonic ε globin is encoded by the HBE1 gene. The two fetal globins, Aγ and Gγ are encoded by the HBG1 and HBG2 genes, respectively. The adult δ globin is encoded by the HBD gene. The adult form of β-globin is encoded by the HBB gene. The difference between the proteins encoded by the HBG1 and HBG2 genes stems from the single amino acid difference between the two fetal genes, glycine in Gγ and alanine in Aγ at amino acid position 136. In addition to the four functional genes in the beta globin cluster there is pseudo beta globin gene (ΨB) that resides between the HBG1 and HBD genes.
More than 350 different mutations have been identified as causing the β-thalassemias. Of these mutations only around 40% are responsible for over 90% of individual cases of β-thalassemia. The molecular causes of β-thalassemia include point mutations (non-deletion β-thalassemias) and gross gene deletions (deletion β-thalassemias).
The non-deletion causes of β-thalassemia included single base substitutions and small insertions or deletions of one to a few bases within the HBB gene or the immediate flanking sequences around the gene. Point mutations that affect β-globin expression are classified by three different categories. One category included mutations in the promoter or 5′-untranslate region (5′-UTR) that result in defective HBB transcription. One category includes mutations that affect mRNA processing and include mutations that involve splice-junction or splicing consensus sequences, polyadenylation sequences, or other 3’ UTR mutations. The third category constitutes mutations that lead to abnormal translation and includes nonsense, frameshift, and initiation codon mutations. The non-deletion mutations represent the major causes of β-thalassemia.
Gross deletion represent rare causes of β-thalassemia. Deletions that have been identified in β-thalassemia that affect only the HBB gene range in size from 67kb to 105kb in size. Two deletions that have been identified result in loss of the 3’ end of the HBB gene but preserve the integrity of the 5’ end the gene. A common cause of β0-thalassemia in Asian Indians involves a deletion of 0.6kb at the 3’ end of the HBB gene. Deletions that remove regions of the β-globin promoter, including the CACCC, CCAAT, and TATA elements, are associated with persistently high levels of HbA2 and variable increases in HbF. The mechanism for the elevated levels of HbA2 and HbF is likely to be loss competition for the upstream β-locus control region (LCR) which results increased interaction of the LCR with the HBG1, HBG2, and HBD genes in cis, thus enhancing their expression.
Clinical and Hematological Findings in β-Thalassemias
Whereas, thalassemia major may result from the homozygous inheritance of a β-thalassemia mutation it is more common that an individual inherits two different β-thalassemias. This latter situation is referred to as a compound heterozygosity. At birth most thalassemia major infants are asymptomatic. However, because fetal hemoglobin (HbF) production declines following birth symptoms of severe anemia will begin to present. If left untreated these children will show a marked impairment in growth rate. As a consequence of the anemia the bone marrow dramatically increases its’ effort at blood production. The cortex of the bone becomes thinned leading to pathologic fracturing and distortion of the bones in the face and skull.
Progressive hepatosplenomegaly is a constant clinical finding as the liver and spleen act as additional sites of blood production. The hepatosplenomegaly leads to leukopenia (decreased white blood cell count) and thrombocytopenia (low platelet count). Recurrent infections are a frequent complication in thalassemia major and are the leading cause of morbidity and mortality in this disease.
Frequent blood transfusions are required to maintain a hemoglobin level of 9 to 11g/dl. However, in the long term these transfusions lead to the accumulation of iron in the organs, particularly the heart, liver and pancreas. Organ failure ensues with death in the teens to early twenties. Iron chelation therapies appear to improve the outlook for β-thalassemia major patients but this requires continuous infusion of the chelating agent.
Thalassemia intermedia is a term used to describe thalassemia patients with anemia and splenomegaly but without the clinical severity of thalassemia major patients. In fact, this syndrome encompasses a range of clinical symptoms from the major form at the severe end to asymptomatic/symptomless. The diagnostic criteria for intermedia is a late presentation and the ability to maintain hemoglobin levels above 6g/dl without transfusion. At the extreme end of the spectrum patients will present with symptoms between 2 and 6 years of age. At the other end of the spectrum are patients who do not present with symptoms until adulthood.
Laboratory Finding in β-Thalassemia
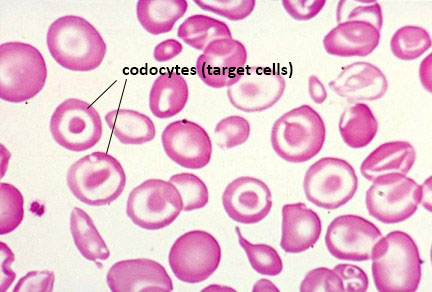
Measurements for serum hemoglobin content, red cell hemoglobin content (mean corpuscular hemoglobin, MCH), and red cell volume (mean corpuscular volume, MCV) are all highly diagnostic for various forms of β-thalassemia major and intermedia. Red blood cell volume is measured in femtoliters (fL) and is termed the mean corpuscular volume, MCV. Normal MCV values are in the range of 82–98 fL. Red blood cell hemoglobin on a per cell basis is assessed as mean corpuscular hemoglobin (MCH) which is normally 26–34 picograms (pg). Red blood cell hemoglobin is measured g/dL where the normal range is between 12 and 16.5 gm/dL depending upon sex. The normal HbA1 level is around 95% of total types of hemoglobin in the post-natal red blood cell, where around 4–5% is HbA2 (consists of the δ-globin protein) and from to 0.5%–2% is HbF (fetal hemoglobin).
Individuals with β-thalassemia major or intermedia have MCV values that are almost always lower than those for individuals with α-thalassemia. The average MCV value for β-thalassemia patients is on the order of 60 ± 3.5 fL. The average MCH value in β-thalassemia is on the order of 19.7 ± 1.3 pg. The average hemoglobin content in the blood in β-thalassemia is 6–7 g/dL.
Laboratory Values Associated with β-Thalassemia Major
| Lab Value | Normal | β-Thalassemia Major |
| MCV (fL) | 82–98 | 60 ± 3.5 |
| MCH (pg) | 26–34 | 19.7 ± 1.3 |
| Hemoglobin (g/dL) | 12–16.5 | 6–7 |
| HbA1 (%) | 95 | 0–5 |
| HbA2 (%) | 2-3 | 2.5–3.5 |
| HbF (%) | 0.5–2 | 30–>95 |